| Dec 05, 2018 | |
Controlling immune response expands the possibilities of therapeutic nucleic acid technologies |
|
| (Nanowerk Spotlight) The idea has been around for a while that selected segments of RNA or DNA could be used therapeutically – so-called therapeutic nucleic acids (TNAs) – to affect gene or cell function. The attraction for researchers is the flexibility that TNAs' versatility, programmability, and modularity affords them and shows a promising route towards treatment for a wide variety of disorders such as cancer, metabolic disorders, viral infections, cardiovascular and inflammatory diseases. | |
| Researchers already have demonstrated that various nucleic acids that are either natural, rationally designed, or selected by directed evolution, can be used to manipulate biological systems and therapeutically utilized to down regulate gene expression (e.g. siRNAs), target receptors (e.g. aptamers), cleave RNAs (e.g. ribozymes), or antagonize transcription (e.g. DNA decoys). | |
| Already, the U.S. Food and Drug Administration (FDA) on August 10th, 2018 approved the very first RNA interference therapeutic agent (Patisiran). | |
| In early clinical trials, some of the proposed TNAs had negative side effects: they provoked (sometimes fatal) reactions from the human body's immune cells (read more: "Defining the immunological effects of nucleic acid nanoparticles"). | |
| Due to the programmability of RNA and DNA, scientists now are able to embed functional assemblies with controllable immunogenic potential into nucleic acid-based nanoparticles (NANPs) to eliminate the immune response and control the timing of their therapeutic activation. | |
| In addition, this new generation of TNAs, made of rationally designed NANPs, have intrinsic immunomodulatory properties on their own which can trigger innate immune responses. The size, sequence, and composition of NANPs contribute to their immunostimulatory properties including the stimulation of production of interferons and pro-inflammatory cytokines. | |
| This novel strategy, developed by Dr. Kirill Afonin’s group at UNC Charlotte and his collaborators, relies on conditional activation of multiple functionalities which is demonstrated by programmable NANPs designed to communicate with each other though sequence complementarity. | |
| This simplified and user-friendly approach allows for conditional activation of RNAi while blocking the transcription of pro-inflammatory genes by forming particular intracellular dsDNAs. | |
| The researchers reported their findings in Nucleic Acids Research("RNA–DNA fibers and polygons with controlled immunorecognition activate RNAi, FRET and transcriptional regulation of NF-κB in human cells"). | |
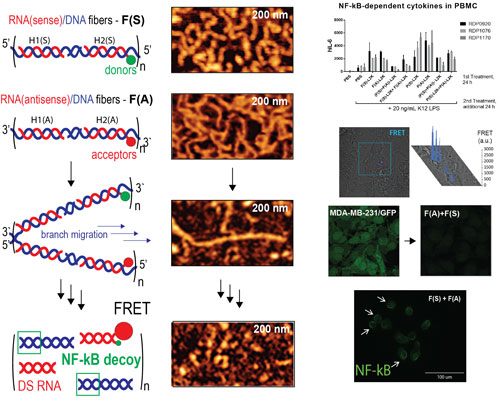 |
|
| Schematics showing re-association of NANPs with subsequent release of RNAi inducers, NF-κB decoys, and activation of FRET (left) and corresponding AFM images, immunological profiles, activation of FRET, RNAi, and NF-κB decoys (right). (click on image to enlarge) | |
| "Our new system is based on a pair of cognate RNA-DNA hybrid NANPs that contains split functional entities; when the cognate pair of NANPs gets in close proximity inside the same cell, their re-association via pre-programmed isothermal strand displacements restores the intended functionalities," Afonin tells Nanowerk. "The design principles are extremely simple with no computational skills and prior experience in RNA or DNA nanotechnology required. The developed system not only allows for a large number of RNAi inducers to be locally activated inside the same cancer cell but also produces fluorescent response and simultaneously releases additional functionalities embedded into the DNA sequences." | |
| The released DNA duplexes become functional and contain NF-κB decoy sequences, which further restrain the immunostimulatory responses, assessed ex vivo in collaboration with Dr. Dobrovolskaia (Nanotechnology Characterization Laboratory, Frederick National Laboratory for Cancer Research). | |
| NF-κB refers to nuclear factor kappa-light-chain-enhancer of activated B cells which is expressed in most mammalian cells and stays inactive in the cytoplasm when bound to inhibitory proteins (IκB). | |
| "Upon stimuli, such as for example exogenous nucleic acids entering the cells, the IκB kinase phosphorylates two serine residues located in an IκB regulatory domain, then the IκB proteins are modified by ubiquitination which degraded by proteasome," Afonin explains. "After the degradation of IκB, the NF-κB complex is then freed to enter nucleus and bind to the κB site of the gene thus “turning on” its expression resulting in pro-inflammatory cytokines production. Besides inflammation, NF-κB is also involved in tumor progression.Controlling the activity of this transcription factor, therefore, represents a therapeutic target in both inflammation and cancer." | |
| "Immunofluorescence analysis revealed a perinuclear accumulation of NF-κB upon lipopolysaccharide (LPS) treatment when the cognate NANPs were co-transfected, suggesting that the translocation of NF-κB was induced by LPS and the NF-κB decoy released as a result of re-association prevent NF-κB entering the nucleus," he continues. "The inhibition of NF-κB functionality was accessed using human peripheral blood mononuclear cells (PBMC), which induce the production of IL-6 and TNFα cytokines in response to LPS." | |
| According to the scientists, the results show that the released NF-κB decoy inhibits the production of LPS-induced IL-6 and TNFα. | |
| Another advantage of the new system is that, by simply flipping the orientation of the interacting DNA parts by 180°, it becomes possible to change the morphology of NANPs from long fibers to closed polygons. | |
| The distinguished shapes of the NANPs lead to particular physiochemical and immunological properties. | |
| "Interestingly, the changes in physicochemical properties were picked up computationally in collaboration with Dr. Dokholayn’s team (Penn State College of Medicine)," notes Afonin. | |
| In this work, the researchers, in collaboration with Drs. Chammas, Saito and Rangel from the Universidade de São Paulo and Instituto do Câncer do Estado de São Paulo, Brazil, Brazil, assessed the therapeutic potential of the NANPs in treating human melanoma cells. | |
| Melanoma is the most dangerous form of skin cancer that begins in the melanocytes that control the pigment in your skin. It is well known for its poor prognosis, ineffective conventional therapies, and recurrences. The discovery that missense mutations in the BRAF gene were present in approximately 60% of melanomas encouraged the development of RAF inhibitors to block the constitutive activation of this gene. | |
| "In 2011, the FDA approved vemurafenib for the treatment of patients with metastatic melanoma harboring BRAFV600E mutation," says Afonin. "The activation of the NF-κB pathway acquired resistance to vemurafenib and the inhibition of this transcription factor increases cell death of vemurafenib-resistant cells. Our model was able to kill two birds with one stone: the released DS RNAs are able to target mutated BRAF and the dsDNA carries NF-κB decoy to inhibit NF-κB functions." | |
| In summary, the responsive behaviors of this novel system are determined by the specific design principles of individual constructs, the type of their assembly, and physicochemical properties. | |
| "Further research on development of smart NANPs could focus on exploring more combinatorial approaches to release multiple functionalities in a more controlled fashion with minimal immunogenicity, which is believed to represent a significant advantage in the field of TNAs," Weina Ke, the paper's first author, concludes. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
