| Posted: May 23, 2006 | |
From lotus leaves to self-cleaning windows |
|
| (Nanowerk Spotlight) Researchers have unlocked the mechanism that makes some leaves either superhydrophobic or hydrophilic, opening the way to creating self cleaning surfaces and interfaces that will not stick. Potential industrial applications are self cleaning windows and windshields, hard disks and magnetic tapes (for data storage) and MEMS and NEMS devices with no stiction issues. | |
| Superhydrophobic materials have surfaces that are extremely difficult to wet with water and therefore are of considerable interest for various industrial applications. The Lotus Effect is based on this principle. Hydrophile on the other hand refers to a physical property of a molecule that can transiently bond with water (H2O) through hydrogen bonding, the exact opposite of hydrophobes. | |
| Professor Bharat Bhushan, director of the Nanotribology Laboratory for Information Storage and MEMS/NEMS at the Ohio State University and his colleague Yong Chae Jung have published the results of their investigations into what makes surfaces hydrophobic by analyzing surface roughness, adhesion and friction data for hydrophobic and hydrophilic leaves on the microscale and nanoscale. The paper, titled "Micro- and nanoscale characterization of hydrophobic and hydrophilic leaf surfaces" was published in the May 16, 2006 online edition of Nanotechnology. | |
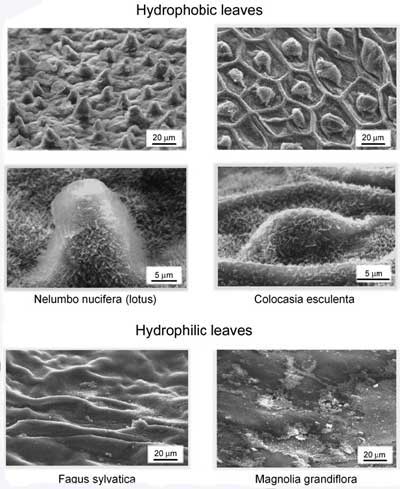 |
Scanning electron micrographs of the relatively rough, water-repellent leaf surface, such as Nelumbo nucifera (lotus) and Colocasia esculenta and the relatively smooth, wettable leaf surfaces, such as Fagus sylvatica and Magnolia grandiflora. (Reprinted with permission from the Institute of Physics) |
| Professor Bhushan explained the background of their research to Nanowerk: "Wetting (hydrophilic property) is characterized by the static contact angle, or simply contact angle, made between a water droplet and a surface, and occurs when the surfaces have a contact angle of less than 90°, whereas if the surface is hydrophobic, the value of the contact angle is greater than 90°. Surfaces with a contact angle between 150° and 180° are called superhydrophobic." | |
| "The contact angle depends on several factors, such as surface energy, roughness, the manner of surface preparation, and surface cleanliness" says Bhushan. | |
| "By removing the surface layer of leaves we found that no wax exists on the hydrophilic leaves but a thin wax film exists on the hydrophobic leaves. The combination of wax and roughness of the leaf is what creates a superhydrophobic surface" Bhushan explains. | |
| In previous work, Bhushan and colleagues focused on microbumps on the surface of leaves to explain their hydrophobic characteristics. They found that there are nanobumps on the top of a microbump and that they may play an important role in surface wettability. Since the morphology and effects of these nanobumps had not been studied before, Bhusan made them the focus of his new study. As a comparison, he also studied hydrophilic leaves to better understand the role of wax and roughness. | |
| What Bhusan found is that the roughness factors of nanobumps are much larger than those of microbumps and are responsible for the increase in contact angle for hydrophobic surfaces. | |
| "For the water droplets sitting on the nanobumps, with decreased spacing between bumps, the probability of air pocket formation increases, and the contact angle increases and contact angle hysteresis decreases" says Bhushan. "In the case of the hydrophilic leaves, while the height of the microbumps can be comparable to that of the hydrophobic leaves, the height of the nanobumps is small. From the analysis of roughness on the micro- and nanoscale, it is found that the nanobumps on the top of the microbumps play a more important role than the microbumps in the hydrophobicity for hydrophobic surfaces." | |
| Bhusan and his team are now working to further develop their computer model to develop optimum surfaces and create them in the lab. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
