| Feb 13, 2019 | |
Defect-free graphene might solve lithium-metal batteries' dendrite problem |
|
| (Nanowerk Spotlight) Rechargeable lithium-ion (Li-ion) batteries are the dominant technology not only for portable electronics but it also is becoming the battery of choice for electric-vehicle and electric-grid energy-storage applications. | |
| In a Li-ion battery, the cathode (positive electrode) is a lithium metal oxide while the anode (negative electrode) is graphite. But researchers are looking for ways to replace graphite with lithium metal as the anode to boost the battery’s energy density. | |
| Lithium metal-based batteries such as Li–sulfur and Li–air batteries have received considerable attention because the packing density of lithium atoms is the highest in its metallic form and Li metal can store 10 times more energy than graphite. | |
 |
|
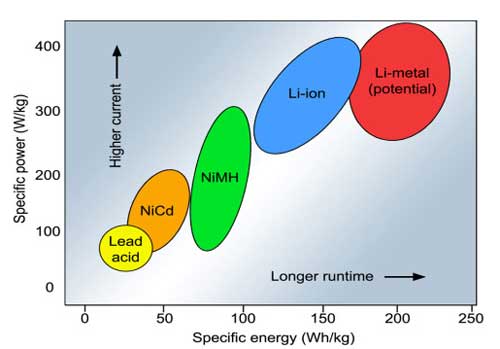
| Specific energy and specific power of rechargeable batteries. Specific energy is the capacity a battery can hold in watt-hours per kilogram (Wh/kg); specific power is the battery’s ability to deliver power in watts per kilogram (W/kg). (Source: Battery University) | |
| However, there are safety and performance concerns for these types of batteries that arise from the formation of dendrites on the metal electrodes; an issue that has been known and investigated since the 1960s. | |
| These dendrites form when metal ions accumulate on the surface of the battery's electrodes as the electrode degrades during the charging process. Dendrites are often responsible for the highly publicized violent battery failures reported in the news. | |
| When these branch-like filament deposits elongate until they penetrate the barrier between the two halves of the battery, they can cause electrical shorts, overheating and fires. They also cause significant cycling efficiency losses. | |
| To avoid dendrites, researchers are experimenting with new battery electrolyte chemistries, new separator technologies, and new physical hosts for the lithium metal. | |
| Carbon hosts, in particular, are very promising, since they may be added to the anode with little additional cost and minimal modification of the manufacturing process and they are becoming an important way to stabilize Li metal anodes. | |
| However, there are seemingly contradictory findings reported in hundreds of prior publications on the subject: The hosts, which are predominantly made from various nanostructured carbons such as graphene, are in some cases very effective in eliminating dendrites. In other cases, they don't work at all, or actually make the dendrite problem worse. | |
| Up to now, design of such host systems has been largely Edisonian: researchers use a trial-and-error approach to find an architecture/structure that works better than the rest. | |
| In new work featured on the front cover of Advanced Energy Materials ("Pristine or Highly Defective? Understanding the Role of Graphene Structure for Stable Lithium Metal Plating"), a research team from Sichuan University in China and Clarkson University in the U.S. have discovered a key design rule for Li metal batteries: If you want to suppress dendrites, you have to use a defect-free host. More generally, carbon defects catalyze dendrite growth in metal anodes. | |
| These findings address the major scientific problem of explaining how the structure and chemistry of the carbon per se affects dendrite growth. | |
 |
|
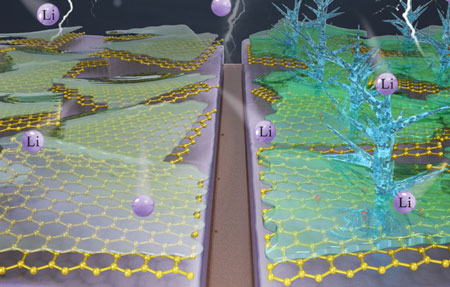
| Left half: Defect-free graphene protecting lithium metal anode from the electrolyte. Right half: Defective graphene catalyzing dendrite growth. (Source: Mitlin Research Group, Clarkson University) | |
| "We discovered a critical and unexpected relationship between the host (graphene) chemical/structural defectiveness and its ability to suppress dendrites," Prof. David Mitlin, who led the work, explains to Nanowerk. "To do this, we created what may be the world's most pure and ordered graphene and compared it to a standard graphene based on reduced graphene oxide. Using such opposite materials, provided unique and fundamental insight into the way lithium dendrites form and grow." | |
| "The key finding, which will rationally guide future lithium battery design efforts, is that the carbon defects are themselves catalytic for dendrite growth," Prof Wei Liu from Sichuan University's Institute of New Energy and Low Carbon Technology, and the paper's first author, points out. "Much of the 'damage' to the anode ultimately responsible for the dendrites occurs even before the battery is fully charged for the first time. Defects in the carbon host corrode the electrolyte at low voltages, leading to early dendrite formation." | |
| The team hypothesized that it was the host structure/chemistry that mattered, but needed to create ideal model systems to test the hypothesis. | |
| Prof. Wei Liu's unique Flow Assisted Sonication (FAS) approach allowed them to create nearly defect-free graphene. Literally, such oxygen-free and structural defect-free graphene has never been synthesized prior by a wet chemistry method. | |
| This graphene is 1-3 atomic layers thick and with only about one and a half percent oxygen. This is much purer than the typical eight percent or more oxygen found in most graphene materials. | |
| "It served as a perfect test bed to explore our hypothesis," says Liu. "Without such a pristine structure, it would not have been possible to obtain the conclusive answers to the dendrite growth problem." | |
| He emphasizes that this in itself is a transformative accomplishment for the carbon and energy communities, since prior only vapor deposition could obtain such ideal defect-free structures. | |
| The team then compared their defect-free graphene to a standard highly defective Hummers graphene baseline found in literature. | |
| "A direct one-to-one comparison allowed us to obtain unique insight into the role of carbon defects on Li dendrite growth," says Mitlin. "A critical new finding is that solid electrolyte interphase (SEI) formation occurring BEFORE metal plating actually dictates dendrites. The fate of the Li metal anode is in effect sealed once the carbon host forms SEI at the initial charge!" | |
| Going forward, the researchers plan to commercialize defect-free graphene host materials for next- generation lithium batteries. They also plan to further understand the complex relationship between carbon defects and metal dendrites by examining carbons with tuned structure/chemistry for lithium, sodium and potassium batteries. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
