| Aug 07, 2019 | |
Polymer encapsulation shields perovskite nanocrystals from degradation |
|
| (Nanowerk Spotlight) Due to their fascinating optoelectronic properties, halide perovskites have attracted tremendous research interest as promising materials for photovoltaics, photodetectors, light-emitting diodes (LEDs), and lasers. | |
| The main attractiveness of perovskite nanocrystals, which were first synthesized in 2014, is their high photoluminescence quantum yield and the possibility to tailor their emission wavelength by selecting the halide in the crystal structure. Numerous synthesis and processing innovations have delivered halide perovskite nanocrystal materials that have been tested in solar cells, solar concentrators, visible light communications, electroluminescent diodes, photodetectors, and photocatalysis. | |
| "Despite recent achievements and a plethora of studies, perovskite nanocrystals still exhibit severe limitations for an unrestricted use in optoelectronic applications," Prof. Dr. Alexander Urban, who leads the Nanospectroscopy Group at the Ludwig-Maximilians-University Munich, tells Nanowerk. "Akin to their bulk counterparts, the nanocrystals degrade due to external environmental influences such as humidity, heat and ultraviolet light illumination." | |
| In new work published in Nano Letters ("Polymer Nanoreactors Shield Perovskite Nanocrystals from Degradation"), Urban and his team report a strategy to synthesize perovskite nanocrystals using diblock copolymer micelles as a growth template. | |
| This work constitutes a new approach for synthesizing perovskite nanocrystals of controllable size and composition with vastly improved resistance to halide ion migration and environmentally induced degradation. | |
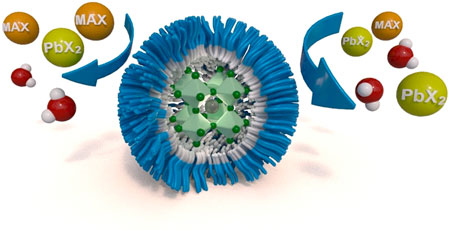 |
|
| Scheme of perovskite nanocrystal encapsulation via diblock copolymer micelles to mitigate moisture-induced degradation and halide ion migration. (Reprinted with permission by American Chemical Society) | |
| Synthesizing the perovskite nanocrystals inside a protective polymer shell shields them from water degradation and halide ion migration yet still allows full control of their properties such as crystal size and emission wavelength. | |
| Since energy transfer between the encapsulated nanocrystals is still possible, this technique allows for their incorporation into electronic devices. | |
| "We showed that during our synthesis the precursor salts diffuse into the cores of the polymer micelles, where halide perovskite nanocrystals spontaneously crystallize," notes Urban. "These exhibit strong photoluminescence as documented by quantum yields of up to 63%." | |
| "Notably" he adds, "the micellar-embedded nanocrystals are vastly superior to standard halide perovskites in terms of stability against humidity." | |
| During long-term testing, the team found that not only were nanocrystals films strongly emissive after more than 200 days of being exposed to ambient conditions but they also exhibited fluorescence after 75 days of complete submersion in water. Additionally, no halide ion migration occurred in these films. | |
 |
|
| Enhanced stability of diblock copolymer-encapsulated perovskite nanocrystals. (a) Temporal development of photoluminescence intensity of perovskite nanocrystal films. Reference MAPI nanocrystals synthesized according to Hintermayr et al. (black curve) degrade in ambient conditions completely within 13 days. In contrast, the encapsulated nanocrystals (green curve) retain nearly 60% of the initial photoluminescence intensity after 130 days. Even completely submersed in water (blue curve), the encapsulated nanocrystals exhibit discernible photoluminescence for over 75 days. (b) Photoluminescence spectra of a film comprising encapsulated MAPBr nanocrystals (green line) and of the same films subjected to aqueous solutions of lead halide (blue points, PbCl2; red points, PbI2). As there is nearly no difference between the spectra, the polymer nanoreactors clearly prevent halide ion migration into or out of the micelles. (Reprinted with permission by American Chemical Society) (click on image to enlarge) | |
| "The excellent stability of the encapsulated nanocrystals combined with their ability to self-assemble in near-perfect films on substrates promises to enable the integration into optoelectronic devices," Urban points out. "F?rster resonance energy transfer (FRET) between nanocrystals of varying bandgap with an extremely high efficiency highlights a possible avenue for realizing this." | |
| "We still need to study the exact formation mechanisms and the specific roles of the polymer components as well as how to enable and optimize charge and/or energy transfer," he concludes. "Nevertheless, we hope that our proposed synthesis technique significantly advances perovskite-based optoelectronics. Furthermore, block copolymers should enable subsequent nanopatterning of thin-films through photolithography and e-beam illumination, greatly expanding the range of possible device structures." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
