| Jun 05, 2020 | |
Filling the knowledge gap about the thermal properties of bismuth antimonide at the nanoscale |
|
| (Nanowerk Spotlight) Bismuth antimonide (BiSb) is an important material in nanoelectronics as a topological insulator – offering unique opportunities to control electric currents and magnetism, and a promising materials for future spintronic applications – and as a thermoelectric material that converts heat into electricity and vice versa. However, some of its thermal properties are still mostly unknown at the nanoscale. | |
| This is an issue because in order for BiSb to exhibit its thermoelectric properties it has to be an alloy i.e. a material where bismuth (Bi) and antimony (Sb) are randomly mixed. But at the nanoscale, it is difficult to mix Bi and Sb, they phase separate very easily i.e. on one side of the nanoparticle you have bismuth and the other side you have antimony; but no mixture with the two chemical elements. | |
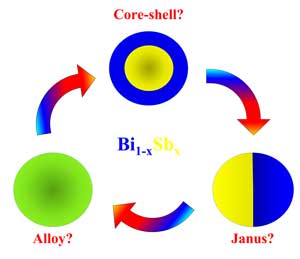
| New findings reported in Journal of Physical Chemistry C ("Chemical Ordering in Bi1-xSbx Nanostructures: Alloy, Janus or Core-Shell?") provide the phase diagram of bismuth antimonide at nanoscale sizes for different types of morphologies like sphere, rod, wire, and film, thereby filling the knowledge gap about the thermal properties of BiSb at the nanoscale. | |
| "By using nanothermodynamics concepts, we newly defined a surface segregation index to easily determine which chemical element segregates preferentially to the surface," Gregory Guisbiers, an Assistant Professor at the University of Arkansas at Little Rock, tells Nanowerk. "The most significant result of our work is that miscibility is not enhanced at the nanoscale but phase separation is!" | |
 |
|
| Chemical ordering in Bi1-xSbx Nanostructures: alloy, Janus or core-shell? (Image courtesy of the researchers) | |
| So far, the phase diagram of BiSb has been totally unknown at the nanoscale, both experimentally and theoretically. This work provides the thermodynamic conditions – temperature and composition – to obtain an alloy of BiSb. Consequently, experimentalists will be able to fabricate alloyed BiSb nanostructures much more easily. | |
| The reason why Guisbiers decided to work with his PhD student Luke D. Geoffrion on this topic is that BiSb is a very interesting material. It was the first experimentally observed topological insulator. It is also a thermoelectric material. | |
| "This material is very complex electronically – its electronic band structure strongly depends on its composition," he elaborates. "Indeed, it changes from semimetal at low Sb concentration (xSb<0.07), to a semiconductor having an indirect bandgap (0.07<xSb<0.09), to a direct bandgap semiconductor (0.09<xSb<0.15), to an indirect bandgap semiconductor again (0.15<xSb<0.22), and finally, it changes back to a semimetal (xSb>0.22) just by enriching the alloy in Sb – but nobody seems to have ever focused on the thermodynamic properties of this material." | |
| However, as the two scientists point out, the electronic properties strongly depend on the chemical ordering of the alloy, which can be determined by knowing the phase diagram of BiSb. So, being aware of the phase diagram of BiSb will help to better design BiSb nanostructures. | |
| The scientific core of these novel findings relates to nano-thermodynamics. A common misconception is that thermodynamics only applies to macroscopic objects; but thermodynamics can still serve at the nanoscale to describe the properties of nano-objects. | |
| "At the nanoscale, we use what we call nano-thermodynamics, i.e. it is still thermodynamics but here we pay extra attention to the surface contribution," Guisbiers explains. "Remember that thermodynamics is built on the conservation of energy which is always true!" | |
| Theoretical knowledge of the phase diagram of BiSb at the nanoscale will help researchers to design and synthesize efficient BiSb nanostructures more effectively i.e. control the chemical ordering of BiSb at the nanoscale. Consequently, the next step of Guisbiers' investigations will be to synthesize BiSb nanoparticles and nanowires based on his team's theoretical calculations. | |
| Another future direction of the team's work is to use nano-thermodynamics on other alloys. However, there are two challenges, as Guisbiers explains: | |
| "The first challenge is that calorimetry is very difficult to perform at the nanoscale. Calorimetry is the technique used to experimentally determine the phase diagram. But, the signal coming from the calorimeter is based on the mass of the material. Therefore, calorimetry is fine to determine the phase diagram of bulk materials but cannot do it for nanomaterials because the mass is too small. Therefore, if we want to determine the phase diagram of materials at the nanoscale we have to rely on theoretical calculations." | |
| "The second challenge" he concludes, "is that most people think that thermodynamics does not apply to the nanoscale, so we are mainly doing educational work showing that thermodynamics does indeed apply to the nanoworld. In the past, we already proved that our calculations were in excellent agreement with some experimental results obtained on gold-copper nanoparticles for example (see: Nanoscale, "Order?disorder phase transitions in Au?Cu nanocubes: from nano-thermodynamics to synthesis")." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
