| Posted: May 05, 2008 | |
EU looks at the safety of nanomaterials in cosmetic products |
|
| (Nanowerk Spotlight) The controversy over the use of nanoparticles in everyday products, such as cosmetics, has been going on for a while now. At best, the evidence is inconclusive – it's too early to say whether there is a risk or not. The cosmetics industry of course argues that their nanoparticle-containing products are perfectly safe because no problem has been reported so far. Consumer, health and environmental groups beg to differ and claim that there is a potential risk because we just don't know enough about this issue and that we rather should err on the side of caution. | |
| The fact is, as a recent report by the European Commission's Health and Consumer Protectorate states, that at present there is inadequate information on hazard identification, exposure assessment, uptake, the role of physico-chemical parameters of nanoparticles determining absorption and transport across membranes in the gut and lungs, the role of physico-chemical parameters of nanoparticles in systemic circulation determining biokinetics and accumulation in secondary target organs, possible health effects, and translocation of nanoparticles via the placenta to the foetus. | |
| That's quite a long list of things we don't know about the fate of nanoparticles introduced into our bodies. The EU report concludes that conventional risk assessment methodologies may be adequate for products that contain soluble and/or biodegradable nanoparticles but not for insoluble and/or biopersistent nanoparticles. | |
| Cosmetic products are primarily intended for use on skin, hair or in the mouth (toothpaste). These products may contain nanoparticulate matter, i.e. with dimensions below 100 nanometers. Cosmetics manufacturers claim that nanoparticles serve various purposes – they enhance the formulation properties and acceptability; have a direct effect on skin and hair, e.g. moisturizing or anti-aging formulations, make-ups and hair-conditioners; or protect the skin e.g. UV-filters in sunscreens. | |
| A crucial factor in assessing possible risks associated with nanoparticles is their possible uptake, i.e. the entrance of a particular nanomaterial into the human body and what subsequently happens to it with regard to accumulation in organs, effects on metabolism, and excretion. | |
| The EU's Scientific Committee on Consumer Products (SCCP) looked at the safety evaluation of nanomaterials for use in cosmetic products and considered the implications on animal testing and whether the previous opinions on nanomaterials currently used in sunscreen products would need to be revised. It reported its findings in March 2008 in a report titled "The Safety of Nanomaterials in Cosmetic Products" (pdf download, 492 KB). | |
| The SCCP report differentiates between soluble and/or biodegradable nanoparticles which disintegrate upon application to skin into their molecular components (e.g. liposomes, microemulsions, nanoemulsions), and and insoluble and/or biopersistent particles (e.g. TiO2, fullerenes, quantum dots). | |
| It finds that for the former, conventional risk assessment methodologies based on mass metrics may be adequate, whereas for the insoluble particles other metrics, such as the number of particles, and their surface area as well as their distribution are also required. | |
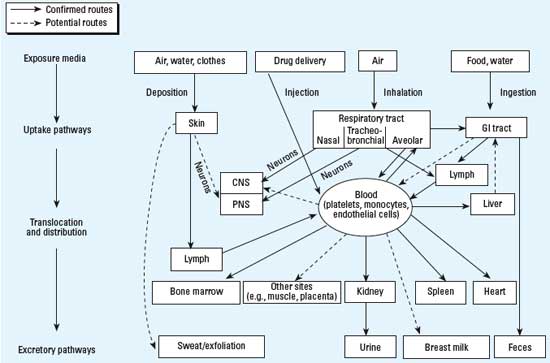
| It is crucial when assessing possible risks associated with nanoparticles to consider their uptake. While many uptake and translocation routes have been demonstrated, others are still hypothetical and need to be investigated. For topical applications, the route of exposure is essentially through the skin but exposure via inhalation, ingestion, conjunctival and mucosal surfaces may sometimes be relevant. | |
 |
|
| While many uptake and translocation routes have been demonstrated, others are still hypothetical and need to be investigated. Largely unknown are translocation rates as well as accumulation and retention in critical target sites and their underlying mechanisms. These as well as potential adverse effects will be largely dependent on physicochemical characteristics of the surface and core of nanosized particles. Both qualitative and quantitative changes in nanosized particles' biokinetics in a diseased or compromised organism need also to be considered. (From: "Toxicology - from coal mines to nanotechnology"; reproduced with permission from Environmental Health Perspectives) | |
| "It is primarily for the insoluble particles that health concerns related to possible uptake arise" the SCCP authors write. "Should they become systemically available, translocation/ transportation and eventual accumulation in secondary target organs may occur. This could become important with repeated application of cosmetic products. Inevitably, insoluble nanoparticles do represent a burden for the environment and a complete life cycle analysis is required." | |
| The report's authors summarize the overall situation as follows: "At present, there is concern about insufficient information in the following areas: | |
|
|
|
| The report takes a critical look at current testing practices and lists several areas of concern: | |
| - Especially relevant for cosmetic applications, the authors points out that in traditional risk assessment, skin penetration studies are carried out using healthy or intact skin. Possible enhanced uptake in case of impaired skin is considered to be covered in the Margin of Safety (MoS). "However, in the case of nanomaterials the conventional MoS may not give an adequate expression of the safety. If there is systemic absorption to vital tissues it may lead to rapid clearance from skin to systemic circulation. It may be anticipated that any systemic absorption is more likely to occur in conditions of abnormal skin e.g. sunburnt, atopic, eczematous, psoriatic skin. There is evidence that physical, in particular mechanical and/or chemical action on the skin may have an effect on nanoparticles penetration." | |
| - There are major data gaps in the assessment of the exposure and the uptake of nanoparticles via dermal absorption, inhalation, oral ingestion and eye contact. The reports looks at available scientific data for all these uptake routes. For instance for dermal exposure it describes the actual situation as follows: | |
| 1) There is evidence of some skin penetration into viable tissues (mainly into the stratum spinosum in the epidermal layer, but eventually also into the dermis) for very small particles (less than 10 nm), such as functionalized fullerenes and quantum dots. | |
| 2) When using accepted skin penetration protocols (intact skin), there is no conclusive evidence for skin penetration into viable tissue for particles of about 20 nm and larger primary particle size as used in sunscreens with physical UV-filters. | |
| 3) The above statements on skin penetration apply to healthy skin (human, porcine). There is an absence of appropriate information for skin with impaired barrier function, e.g. atopic skin or sunburned skin. A few data are available on psoriatic skin. | |
| 4) There is evidence that some mechanical effects (e.g. flexing) on skin may have an effect on nanoparticle penetration. | |
| 5) There is no information on the transadnexal penetration for particles under 20 nm. Nanoparticles of 20 nm and above penetrate deeply into hair follicles, but no penetration into viable tissue has been observed. | |
| - Although the basic requirements of testing the mutagenicity/genotoxicity of nanoparticles are similar to those of other particulate materials, the specific characteristics of nanoparticles may require further considerations. The present validated in vivo genotoxicity tests, however, do not cover the expected target organs of nanoparticles (particularly the respiratory tract) and have not been validated with reference substances including nanomaterials of relevance for cosmetics. | |
| - All in vivo and in vitro risk assessment methods for nanomaterials are still in the research phase. Although some validated in vitro methods do exist they have never been validated with nanomaterials as reference compounds. | |
| - Although animal testing can be largely reduced for skin penetration studies, they are essential for translocation and accumulation studies as well as for chronic toxicity studies. | |
| - The SCCP considers it necessary to review the safety of nanosized titanium dioxide in the light of recent information and to consider the influence of physiologically abnormal skin and the possible impact of mechanical action on skin penetration. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
