| Oct 28, 2020 | |
Designing nucleic acid nanotechnology for targeted immunostimulation in human cells |
|
| (Nanowerk Spotlight) Nucleic acid nanoparticles (NANPs) are a new type of nanomaterials that are exclusively made of rationally designed nucleic acids such as RNA, DNA and their chemical analogs. Although these NANPs are quite small ranging from 10-100 nm, they have already demonstrated an extremely large therapeutic potential. | |
| It is now becoming more and more apparent that nucleic acids offer an ideal building material for the development of therapeutic nucleic acids because they are biocompatible and can be programmed as or functionalized with antisense oligonucleotides, small interfering RNA (siRNAs), microRNAs (miRNAs), aptamers, and decoy sequence1–6. | |
| Currently, there are only a dozen of successful examples of therapeutic nucleic acids approved for clinical use by the U.S. Food and Drug Administration7–14. The reason for this is that the clinical application of therapeutic nucleic acids is often limited by factors such as their chemical instability, targeted cellular delivery, and off-target inflammatory immune responses that are detrimental to the host15–17. The same problems also preclude more rapid clinical translation of NANPs. | |
| In a paper published in Nucleic Acid Research ("The immunorecognition, subcellular compartmentalization, and physicochemical properties of nucleic acid nanoparticles can be controlled by composition modification"), researchers Dr. Kirill Afonin, Dr. Brittany Johnson, and collaborators, document that nucleic acid composition can be designed for enhanced stability, targeted cellular delivery, and optimal activation or abrogation of immune responses. | |
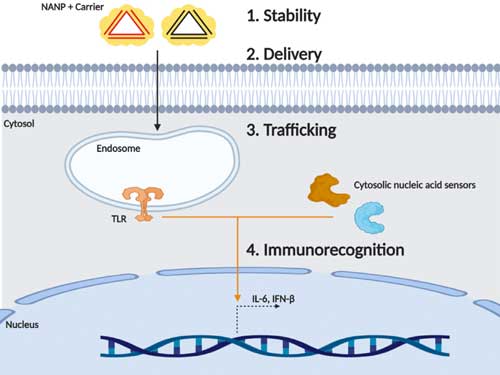
| This research examines a focused panel of NANPs that maintain the same connectivity, shape, size, charge, and sequence to evaluate specifically the role of nucleic acid composition on temperature and enzymatic stability, delivery efficiency and localization, and immune stimulation. | |
 |
|
| The chemical composition of NANPs defines their physicochemical properties while tuning immune recognition and cellular compartmentalization. (Oxford University Press, Creative Commons CC BY) | |
| Consistent with previous data, incorporation of RNA nucleic acid strands increased thermal stability due to more favorable base-pair stacking18,19. However, RNA NANPs are highly susceptible to degradation by nucleases20,21. | |
| To alleviate this instability, incorporation of nucleic acid strands that contain 2' fluorinated pyrimidines (uracil and cytosine) can be employed as a simple way to reduce NANPs digestion by nucleases, thus providing a means to overcome enzymatic instability. | |
| The negative charge of NANPs can limit delivery to host cells due to charge repulsion6. Here lipid-based carriers were used to successfully deliver NANPs. Certain NANP carrier complexes displayed increased delivery efficiency, specifically RNA NANPs with 2' fluorine modified nucleic acids strands yielded the highest delivery efficiency. | |
| Additionally, the scientists observed that while all NANPs traffic from an endosomal compartment to the cytosol, nucleic acid composition impacted the degree of enrichment to these compartments providing a means for target delivery of NANPs on a subcellular level. | |
| Notably, the team examined six NANPs combined with two different lipid-based carrier – but an almost endless number of NANPs can be constructed and there are many other potential carrier options providing a vast library to tailor targeted NANP delivery. | |
| Off-target immune stimulation occurs because host cells possess nucleic acid sensors in the endosomal compartment and cell cytosol to detect foreign or damage-associated nucleic acids22,23. These nucleic acid sensors have the potential to detect NANPs based on identification of specific ligand characteristics22,23. | |
| Identification of NANPs can then trigger production of immune mediators with either detrimental or potentially beneficial effects17,24–28. Here, the authors demonstrate that nucleic acid composition of NANPs can be designed to avoid immune detection or elicit production of beneficial immune mediators. | |
| The team notes that their data indicates that RNA NANPs are more immunostimulatory when compared to the same NANPs but made of DNA that showed to be immunoquiescent. As such, DNA NANPs provide a platform that can deliver functional groups while triggering minimal immune activation. | |
| In contrast, RNA NANPs provide a promising platform for development of pan-antivirals, vaccine adjuvants, and cancer therapeutics that purposefully activate immune sensors to trigger beneficial immune responses. | |
| Here the team demonstrates that RNA NANPs activate the endosomal sensor, toll-like receptor 7 (TLR7) and the cytosolic sensor, retinoic acid inducible gene-I (RIG-I). Incorporation of 2' fluorine modified nucleic acid strands can be employed to prevent TLR7 responses but allow RIG-I mediated responses thereby targeting specifically RIG-I. | |
| Interestingly, RIG-I can also be activated indirectly using DNA NANPs with 2' fluorine modified nucleic acid strands due to RNA polymerase III conversion of these NANPs to an RNA ligand for RIG-I. | |
| "Collectively, our data indicates nucleic acid composition can control NANP thermo and enzymatic stabilities, targeted delivery, and immunostimulatory properties allowing for the rational design of therapeutic NANPs," the researchers conclude. | |
References |
|
| 1. Catuogno,S., Esposito,C.L., Condorelli,G. and de Franciscis,V. (2018) Nucleic acids delivering nucleic acids. Adv. Drug Deliv. Rev., 134, 79–93. | |
| 2. Alvarez-Salas,L. (2008) Nucleic Acids as Therapeutic Agents. Curr. Top. Med. Chem., 8, 1379–404. | |
| 3. Panigaj,M., Johnson,M.B., Ke,W., McMillan,J., Goncharova,E.A., Chandler,M. and Afonin,K.A. (2019) Aptamers as Modular Components of Therapeutic Nucleic Acid Nanotechnology. ACS Nano, 13, 12301–12321. | |
| 4. Afonin,K.A., Viard,M., Koyfman,A.Y., Martins,A.N., Kasprzak,W.K., Panigaj,M., Desai,R., Santhanam,A., Grabow,W.W., Jaeger,L., et al. (2014) Multifunctional RNA nanoparticles. Nano Lett., 14, 5662–5671. | |
| 5. Stewart,J.M., Viard,M., Subramanian,H.K.K., Roark,B.K., Afonin,K.A. and Franco,E. (2016) Programmable RNA microstructures for coordinated delivery of siRNAs. Nanoscale, 8, 17542–17550. | |
| 6. Jasinski,D., Haque,F., Binzel,D.W. and Guo,P. (2017) Advancement of the Emerging Field of RNA Nanotechnology. ACS Nano, 11, 1142–1164. | |
| 7. Hutcherson,S.L. and Lanz,R. (2002) A randomized controlled clinical trial of intravitreous fomivirsen for treatment of newly diagnosed peripheral cytomegalovirus retinitis in patients with aids. Am. J. Ophthalmol.. | |
| 8. Ng,E.W.M., Shima,D.T., Calias,P., Cunningham,E.T., Guyer,D.R. and Adamis,A.P. (2006) Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nat. Rev. Drug Discov.. | |
| 9. Raal,F.J., Santos,R.D., Blom,D.J., Marais,A.D., Charng,M.J., Cromwell,W.C., Lachmann,R.H., Gaudet,D., Tan,J.L., Chasan-Taber,S., et al. (2010) Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of LDL cholesterol concentrations in patients with homozygous familial hypercholesterolaemia: a randomised, double-blind, placebo-controlled trial. Lancet. | |
| 10. Stein,C.A. (2016) Eteplirsen approved for duchenne muscular dystrophy: The FDA faces a difficult choice. Mol. Ther.. | |
| 11. Hua,Y., Sahashi,K., Hung,G., Rigo,F., Passini,M.A., Bennett,C.F. and Krainer,A.R. (2010) Antisense correction of SMN2 splicing in the CNS rescues necrosis in a type III SMA mouse model. Genes Dev.. | |
| 12. Splawn,L.M., Bailey,C.A., Medina,J.P. and Cho,J.C. (2018) Heplisav-B vaccination for the prevention of hepatitis B virus infection in adults in the United States. Drugs of Today. | |
| 13. Gales,L. (2019) Tegsedi (Inotersen): An antisense oligonucleotide approved for the treatment of adult patients with hereditary transthyretin amyloidosis. Pharmaceuticals. | |
| 14. Adams,D., Gonzalez-Duarte,A., O'Riordan,W.D., Yang,C.-C., Ueda,M., Kristen,A. V., Tournev,I., Schmidt,H.H., Coelho,T., Berk,J.L., et al. (2018) Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. N. Engl. J. Med.. | |
| 15. Afonin,K.A., Dobrovolskaia,M.A., Church,G. and Bathe,M. (2020) Opportunities, Barriers, and a Strategy for Overcoming Translational Challenges to Therapeutic Nucleic Acid Nanotechnology. ACS Nano, 14, 9221–9227. | |
| 16. Chandler,M., Panigaj,M., Rolband,L.A. and Afonin,K.A. (2020) Challenges in optimizing RNA nanostructures for large-scale production and controlled therapeutic properties. Nanomedicine, 15, 1331–1340. | |
| 17. Dobrovolskaia,M.A. and McNeil,S.E. (2015) Immunological and hematological toxicities challenging clinical translation of nucleic acid-based therapeutics. Expert Opin. Biol. Ther., 15, 1023–48. | |
| 18. Bui,M.N., Brittany Johnson,M., Viard,M., Satterwhite,E., Martins,A.N., Li,Z., Marriott,I., Afonin,K.A. and Khisamutdinov,E.F. (2017) Versatile RNA tetra-U helix linking motif as a toolkit for nucleic acid nanotechnology. Nanomedicine Nanotechnology, Biol. Med., 13, 1137–1146. | |
| 19. Hong,E., Halman,J.R., Shah,A.B., Khisamutdinov,E.F., Dobrovolskaia,M.A. and Afonin,K.A. (2018) Structure and Composition Define Immunorecognition of Nucleic Acid Nanoparticles. Nano Lett., 18, 4309–4321. | |
| 20. Bramsen,J.B. and Kjems,J. (2011) Chemical modification of small interfering RNA. Methods Mol. Biol., 721, 77–103. | |
| 21. Sioud,M. (2006) Single-stranded small interfering RNA are more immunostimulatory than their double-stranded counterparts: A central role for 2'-hyroxyl uridines in immune responses. Eur. J. Immunol., 36, 1222–1230. | |
| 22. Wu,J. and Chen,Z.J. (2014) Innate Immune Sensing and Signaling of Cytosolic Nucleic Acids. Annu. Rev. Immunol., 32, 461–488. | |
| 23. Kawasaki,T. and Kawai,T. (2014) Toll-like receptor signaling pathways. Front. Immunol., 5. | |
| 24. Beutner,K.R., Spruance,S.L., Hougham,A.J., Fox,T.L., Owens,M.L. and Douglas,J. (1998) Treatment of genital warts with an immune-response modifier (imiquimod). J. Am. Acad. Dermatol., 38, 230–239. | |
| 25. Geisse,J., Caro,I., Lindholm,J., Golitz,L., Stampone,P. and Owens,M. (2004) Imiquimod 5% cream for the treatment of superficial basal cell carcinoma: Results from two phase III, randomized, vehicle-controlled studies. J. Am. Acad. Dermatol., 50, 722–733. | |
| 26. Goulet,M.L., Olagnier,D., Xu,Z., Paz,S., Belgnaoui,S.M., Lafferty,E.I., Janelle,V., Arguello,M., Paquet,M., Ghneim,K., et al. (2013) Systems Analysis of a RIG-I Agonist Inducing Broad Spectrum Inhibition of Virus Infectivity. PLoS Pathog., 9. | |
| 27. Martinez-Gil,L., Goff,P.H., Hai,R., Garcia-Sastre,A., Shaw,M.L. and Palese,P. (2013) A Sendai Virus-Derived RNA Agonist of RIG-I as a Virus Vaccine Adjuvant. J. Virol., 87, 1290–1300. | |
| 28. Beljanski,V., Chiang,C., Kirchenbaum,G.A., Olagnier,D., Bloom,C.E., Wong,T., Haddad,E.K., Trautmann,L., Ross,T.M. and Hiscott,J. (2015) Enhanced Influenza Virus-Like Particle Vaccination with a Structurally Optimized RIG-I Agonist as Adjuvant. J. Virol., 89, 10612–10624. | |
| Provided by the University of North Carolina Charlotte as a Nanowerk exclusive. | |
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
