| Oct 29, 2020 | |
High-entropy alloy nanoparticles show excellent resistance to oxidation |
|
| (Nanowerk Spotlight) High-entropy alloys (HEAs), which are formed by combining nearly equal parts of several – usually five or more – primary metals, are an emerging class of advanced materials that hold great potential for creating materials with superior mechanical, thermal, and catalytic properties. Another attraction of HEAs is that they can create effective alternatives to materials that are scarce, hazardous, expensive, or subject to international restrictions or conflict. | |
| Similar to conventional alloys, industrial operation environments for HEAs usually involve critical conditions such as exposure to high temperature, oxidizing/reducing gases, and acidic and chloride-containing solutions. | |
| Several studies have been devoted to understanding the corrosion behaviors of HEAs such as oxidation in air and oxygen-containing atmospheres. Nevertheless, there currently is little knowledge of how HEA materials, especially in nanoparticulate form, behave under critical conditions involving oxidizing gases due to the limited characterization methods available for dynamic events. | |
| A new research report in ACS Nano ("In Situ Oxidation Studies of High-Entropy Alloy Nanoparticles") offers key insights into how HEA nanoparticles behave under high-temperature oxidizing environment and sheds light on future design options of highly stable alloys under complex service conditions. This work has been a multidisciplinary and multi-institutional effort led by the University of Illinois at Chicago in collaboration with Argonne National Laboratory, University of Pittsburgh, University of California Riverside, and Northwestern University. | |
 |
|
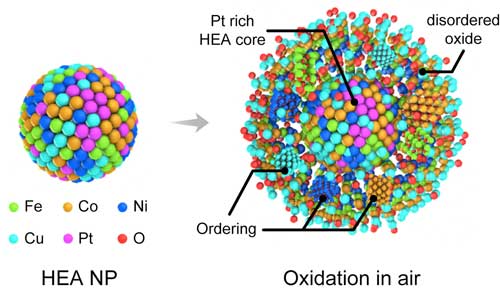
| Schematic illustration of the oxidation process of high-entropy alloy nanoparticles. (Reprinted with permission by American Chemical Society) (click on image to enlarge) | |
| "Traditional metals or alloys are believed to form crystalline oxides based on the composition and oxidation conditions, but when five or more principle elements are involved in a single phase, novel behavior during oxidation may exist due to the built-in high entropy," Boao Song from the Department of Mechanical and Industrial Engineering at the University of Illinois at Chicago, and the paper's first author, tells Nanowerk. "We were intrigued to find out that the transition metals in HEA nanoparticles can co-segregate into the oxide layer and form a disordered phase that can slow down the whole oxidation process." | |
| For the first time, this work directly captured the whole oxidation process of HEA nanoparticles in real-time and at the nanoscale. The advanced in situ transmission electron microscopy (TEM) technique used by the UIC team allows real-time imaging and analytical methods, and thus provides clear evidence of the co-segregation of transition metals during the oxidation process. | |
| It also confirms the much slower oxidation kinetics of HEA nanoparticles. These findings suggest the possibility of utilizing these high-entropy alloys for applications where high thermal stability and oxidation resistance are required. | |
 |
|
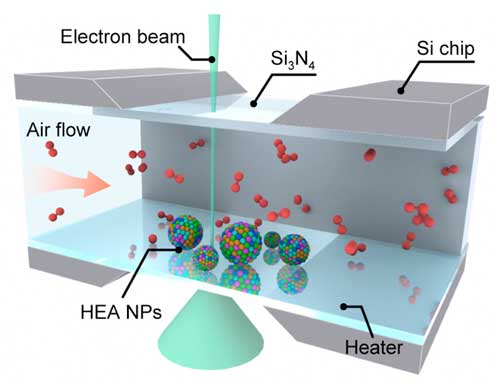
| Schematic of the in situ gas-cell (S)TEM device to study the oxidation of HEA nanoparticles. (Reprinted with permission by American Chemical Society) | |
| The researchers managed to successfully capture the high-temperature oxidation of FeCoNiCuPt HEA nanoparticles in real-time by utilizing the advanced in situ gas-cell transmission electron microscopy (TEM) technique combined with density functional theory (DFT) calculations. | |
| "We found that the oxidation kinetics of HEA nanoparticles are significantly slower than those of monometallic and bimetallic alloys," says Song. "Combining in situ energy dispersive spectroscopy (EDS) and electron energy loss spectroscopy (EELS) analyses, we confirmed that the oxidation of HEA nanoparticles are governed by Kirkendall effects displaying outwards segregation of Fe, Co, Ni and Cu." | |
| "The present study is crucial for understanding HEA behavior in oxidizing environments and provides insights into designing high-temperature-resistant materials, durable catalysts, and corrosion-resistance alloys for various applications,"Song concludes. "Still, there are a lot of undiscovered phenomena with regards to HEAs and a lack of well-established theories to explain them. For example, the mechanism that controls the oxidation process and why the differences are so huge compared to monometallic nanoparticles is still unknown." | |
| So it appears that the team has their work cut out for them. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
