| Nov 10, 2020 | |
Surface chemistry of engineered nanoparticles allows to differentiate cancer stem cells |
|
| (Nanowerk Spotlight) Cancer stem cells (CSCs), also called tumor-initiating cells, are elusive and rare cells that can cause cancer to come back even years after the initial tumor has been successfully treated. Although CSCs represent only a tiny fraction of cells in a tumor it only takes one to seed a new tumor when it splits into a cancer cell and another stem cell. | |
| With their ability to resist conventional chemotherapy drugs, CSCs are really difficult to kill and they are considered to be key drivers in metastasis, the spread of cancer via the blood stream. | |
| The underlying cause for this is a process known as mesenchymal-to-epithelial transition (MET) whereby CSCs differentiate and become regular cancer cells. Conversely, cancer cells can also gain stem cell properties to become more aggressive by undergoing epithelial-to-mesenchymal transition (EMT). | |
| Researchers have hypothesized that therapeutic agents that promote differentiation could limit the occurrence of EMT and with it the acquisition of stem cell properties by cancer cells. | |
| If the frequency of CSCs could be diminished, tumors would be rendered less aggressive and more responsive to conventional therapy. This approach is known as differentiation therapy and has considerable therapeutic potential. | |
| "Differentiation therapy presents a potential strategy for 'defanging' CSCs," Vincent Rotello, the Charles A. Goessmann Professor of Chemistry and a University Distinguished Professor at the University of Massachusetts at Amherst, tells Nanowerk. "Specifically, the use of nanoparticles to differentiate CSCs provides a new therapeutic strategy against these particularly dangerous cancer cells." | |
| The crucial issue, of course, is to find the nanoparticles that are effective as differentiating agents. | |
| In a recent paper in ACS Nano ("Differentiation of Cancer Stem Cells through Nanoparticle Surface Engineering"), Rotello and his group report the integrated use of nanoparticle engineering and hypothesis-free sensing to identify nanoparticles capable of efficient differentiation of CSCs into non-CSC phenotypes. | |
| "Limitations in screening and platform design space have hindered the exploration of nanomaterial-based CSC differentiation, and as such, there is a clear need for methods that feature greater control over chemical diversity and can simultaneously increase throughput," says Rotello. | |
| The team's novel approach uses the precise level of structural control afforded by gold nanoparticles to systematically investigate the effects of nanoparticle surface chemistry on CSC differentiation. | |
| Coupling the gold nanoparticles with a hypothesis-free, array-based nanosensor platform allows access to the resulting cell phenotype in a matter of minutes – a feature highly desirable for high-throughput and high-content screening. | |
 |
|
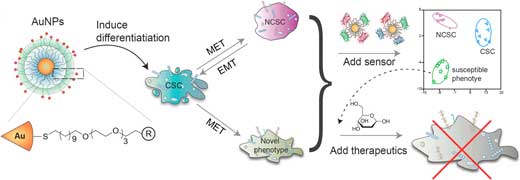
| Schematic illustration of the integration of nanoparticle design and hypothesis-free sensing. Chemical structure of the 2 nm core diameter gold nanoparticles (AuNPs) used to stimulate CSCs is shown on the left. After AuNP treatment, cells were washed and incubated with the nanosensor to rapidly collect phenotypic information. An unreported phenotype identified through the sensing screen was further subjected to drug treatment to see if it was more sensitive to chemotherapy. (Adopted and reprinted with permission by American Chemical Society) (click on image to enlarge) | |
| "Our array-based sensing uses a signature-based method to profile an entire matrix of bioanalytes, effectively circumventing the bottleneck of receptor specificity as demonstrated through discrimination of CSCs from non-CSCs," Rotello points out. "With our platform we successfully identified an altered unreported phenotype that is more susceptible to traditional chemotherapeutic treatment. The resulting cells presented an increased level of reactive oxygen species, which could be responsible for sensitizing CSCs to drug treatment." | |
| "The nanoparticle used in our study provides a lead for designing more effective CSC-targeted therapeutics," he concludes. "The mechanistic studies backing up the research also provide new insight into mechanisms that can be accessed to target CSCs." | |
| There will be challenges in moving these results from cell studies into animals. The nanoparticles used, however, have excellent properties for in vivo use. | |
| Going forward, the researchers are currently developing new, more efficient nanoparticles and will be testing them in animal models soon. They are also continuing their fundamental studies into the mechanism(s) of CSC differentiation. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
