| Posted: Jun 15, 2006 | |
Natural polymer nanofibers for cartilage repair |
|
| (Nanowerk Spotlight) Scientists at the University of Washington are developing natural polymer based nanofibers using electrospinning to mimic the native extracellular matrix of cartilage in terms of microstructure, mechanical properties, and chemical composition. This reesearch holds great implication for the generation of functional cartilage tissues to help the millions of people who suffer from degeneration of articular cartilage due to primary osteoarthritis or trauma. | |
| Damaged articular cartilages ordinarily demonstrate a very limited capability for self-healing, functional restoration of diseased or damaged articular cartilage is a major clinical challenge. There have been a number of successful approaches to tissue engineered cartilage, including the use of natural and synthetic biomaterial scaffolds, allogeneic and autologous sources of mature chondrocytes, and chondroinductive growth factors. Although recent progress has been made in engineering cartilage of various shapes and sizes for cosmetic purposes, current treatments for cartilage repair are less than satisfactory, and rarely restore full function or return the tissue to its native state. | |
| Nanofibrous matrices are introduced as scaffolds that may have a better structural resemblance to target tissues than their bulk counterparts, because major components in tissues are nanofeatured structures and cells appear to attach and proliferate better on nanofeatured structures than on bulk materials. This concept has been known for years in the scientific community, but only when the materials can be manipulated at the nanoscale, it sheds light on tissue engineering. | |
| There is a rapidly growing interest in synthesis of natural polymer based nanofibers because of their proven biocompatibility and resorbable biodegradation products. Advantageous attributes of natural polymers include hydrophilicity, nontoxicity, less immune reaction, as well as enhanced cell adhesion and proliferation. However, fabrication of natural polymer nanofibers by electrospinning is challenging. This is because the gelation of chitosan- and alginate start to occur at a very low polymer concentration at which the solution contains insufficient material to generate fibrous structures, and rather, sprayed droplets or a structure with short fibers embedded with beads are obtained. At slightly higher polymer concentrations, the solution becomes so dense that renders the solution un-injectable. | |
| Chitosan and alginate, two abundant natural polymers, have been widely used in tissue engineering, but none had been fabricated into nanostructured matrices until in recent two years. Professor Miqin Zhang from the Material Science & Engineering department at the University of Washington, and members of her group reported the successful fabrication of chitosan based nanofibers ("Electrospun chitosan-based nanofibers and their cellular compatibility") as well as the first fabrication of alginate-based nanofibers ("Alginate-Based Nanofibrous Scaffolds: Structural, Mechanical, and Biological Properties"). | |
| Zhang explains her group's work to Nanowerk: "We fabricate chitosan- and alginate- based nanofibrous matrices to closely mimic the extracellular matrix (ECM) of articular cartilage that primarily consists of type II collagen and proteoglycans (glycosaminoglycan, GAG). GAGs are considered to play a significant role in modulating chondrocyte cell morphology, differentiation, and function. Both chitosan and alginate can be considered to be an excellent proxy for cartilage GAGs." | |
| Figs. 1A, B and C show the exemplary images of alginate based nanofibers fabricated by electro- spinning from solutions of 80 wt% alginate, 20 wt% PEO. The nanofibers have an average diameter of ∼75 nm and a narrow size distribution. The chondrocytes cultured on alginate nanofibers adhered and proliferated well, and appeared round in shape and maintained their characteristic phenotypes (Fig. 1B). Notably, unlike bulk alginate scaffolds which usually require pre-coated adhesion proteins such as fibronectin or RGD peptides to facilitate cell adhesion, the alginate based nanofibrous matrix does not need such pretreatment. | |
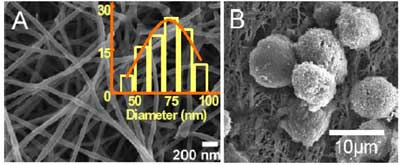 |
Fig. 1. SEM images of alginate-based nanofibers and size distribution (inset) (A). Chondrocytes grown on alginate based nanofibers (B). (Source: Miqin Zhang, University of Washington) |
| Figs. 2A, B and C show the exemplary images of chitosan based nanofibers fabricated by electrospinning polymer solutions of chitosan (80 wt%) and PEO (20 wt%). PEO is an FDA approved biocompatible polymer for internal consumption and injection in a variety of foods. The nanofibers have average diameters of 38 ±8 nm (A) when deposited randomly and 62 ±12 nm (B) when collected on a rotating collector. Figs. 1D and E show the SEM images of osteoblast (MG-63) and chondrocytes (HTB-94) grown on the chitosan nanofibers, respectively. Osteoblasts adhered well on the nanofibrous matrix and exhibited discrete filopodia and long and numerous microvilli on their surfaces. Chondrocytes have the characteristic morphology of a round shape, indicating that the nanofibrous matrix maintained the phonotype of chondrocytes. | |
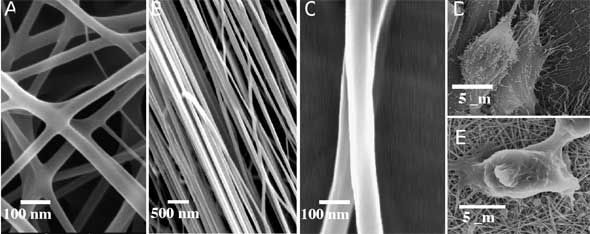 | |
| Fig. 2. SEM images of electrospun chitosan-based nanofibers collected from (A) a stationary collector and (B) a collector rotating at 2000 rmp. (C): Nanofibers shown in (B) at higher magnification. (D) and (E): Images of osteoblasts (MG-63) and chondrocytes (HTB-94) grown on the nanofibrous matrix 5 days after cell culture. (Source: Miqin Zhang, University of Washington) | |
| Zhang explains the significance of her group's findings: "These nanostructured scaffolding materials may potentially change the way we heal joint osteoarthritis and cartilage loss clinically in the future. The ability to generate nanofibrous matrices from natural polymers may provide virtually unlimited resources for development of tissue compatible scaffolds for functional restoration of damaged or dysfunctional tissues, which currently relies mainly on the autograft and allograft?the surgical procedures facing challenges of limited resources, and risk of disease transmission or viral transfection. " | |
| She points out that, in addition to the production of these clinically applicable materials, the research in this area would offer insights to the interactions between materials and biological systems at the nanoscale, and the technology developed and knowledge gained would provide a foundation that can be expanded upon to many applications in biomaterials, tissue engineering, drug delivery, material-biological molecular interfaces, and other aspects of heath care. | |
| "We will fabricate nanofibrous natural polymer matrices with improved physical and biological properties" Zhang says. "In vitro cellular and molecular events of chondrocyte interactions with synthesized nanofiber matrix will be systematically studied to identify the proper material nano-structures (i.e., porosity, pore size, shape, nanofiber size and distribution) that provides the optimal environment for cell attachment and proliferation, and phenotype expression. To expedite the tissue regenerative process, the chondrocyte cells or stem cells and growth factors will be also incorporated in nanofiberous matrices for both in vitro and in vivo studies. Strain patterns during ingrowth into the scaffolds will be recorded to correlate with quantitative histomorphometry and radiography to define relationships between loading and tissue structure. This information will be used to develop cell culture loading systems to produce functional cartilage construct." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
