| Dec 29, 2020 | |
A lithium-air battery with long cycle life and low overpotentials |
|
| (Nanowerk Spotlight) One of the bottlenecks in widespread implementation of sustainable energy technologies are highly efficient energy storage systems. Lithium-ion batteries (LIBs) are the prevailing solution for today's electronic devices, from consumer gadgets to medical devices, electric vehicles, even satellites. The main reason for the domination of LIB technology in many application areas is that it has the highest electrical storage capacity with respect to its weight. | |
| LIBs generally contain an energy capacity of 100–200 Wh/kg. This allows most electric cars to travel upwards of 300-400 kilometers on a single change. However, despite the high energy density of LIBs compared to other kinds of batteries, they are still around a hundred times less energy dense than gasoline (which contains 12700 Wh/kg by mass or 8760 Wh/L by volume). That means that gasoline-powered engines are gaining higher thermal efficiencies, allowing for fuel efficiencies upwards of 6-7 L/100km. On a 60-liter tank, this allows for more than 800-1000 km of range, easily doubling, or even tripling that of the average electric car. | |
| Although LIBs are continuing to achieve higher energy densities, various research studies are indicating that max theoretical energy limits (estimated at 400-500 Wh/kg) are in sight. | |
 |
|
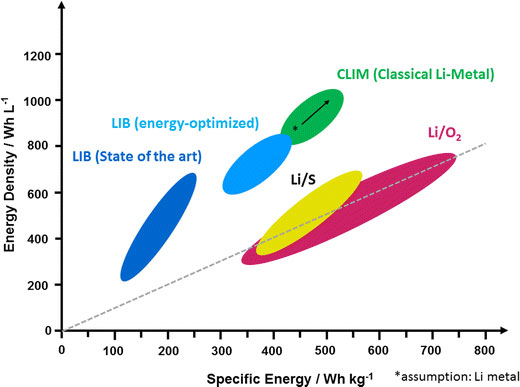
| Energy density vs. specific energy plot of today’s LIBs (dark blue) in comparison to energy-optimized LIBs (light blue), classical Li-metal batteries (CLIMs; green) and post-lithium ion technologies such as lithium/sulfur (Li/S) as well as lithium/oxygen batteries (Li/O2) on the cell level. The dotted gray line represents the parity of energy density and specific energy (Wh/L = Wh/kg). (Source) | |
| In the search for battery technologies that one day could replace LIBs and meet power demands within the size and cost constraints of car makers, aerospace and other industries, researchers are exploring lithium-air batteries (LABs). | |
| Oxygen is an advantageous battery storage material as it is freely available from the air and does not need to be carried with the other battery components. Unlike the lithium-ion batteries used today, lithium–air batteries do not require metal oxide cathodes to produce electrochemical power, instead generating power from reactions with oxygen in the atmosphere. | |
| Unfortunately, today's lithium-air batteries have some pretty serious drawbacks: They waste much of the injected energy as heat and degrade relatively quickly, lasting only a few charging cycles. | |
| One of the major issues for Li–air batteries is that the developed catalysts exhibit sluggish activity for both oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) or only remain active for one of the reactions (different ORR/OER rates). This can result in high overpotentials – excess energy above its thermodynamic value (2.96 V) – required to form and decompose lithium peroxide (Li2O2) at the cathode during discharge (ORR) and charge (OER) processes, respectively. | |
| This technology has been extensively studied by different research groups. Numerous metal catalysts like platinum, gold, and ruthenium, as well as non-metallic catalysts such as transition-metal oxides, transition-metal dichalcogenides, and carbon-based catalysts, have been employed to resolve this issue; however, no major breakthrough has been reported to date. | |
| "In a lithium-air cell, energy is stored in the form of covalent bonds rather than via intercalation happening in LIBs – more specifically, the chemistry of these systems is governed by two catalytic reactions: the OER and the ORR during the charge and discharge cycles, respectively," Dr. Mohammad Asadi, Assistant Professor of Chemical and Biological Engineering at the Illinois Institute of Technology, tells Nanowerk. "The effect of coupling these reactions together accounts for a much higher energy capacity compared to their lithium-ion counterparts; however, their practical application is hampered by low cyclability and poor energy efficiency. These are mainly owing to the poor catalytic activity of electrocatalysts to drive the OER and ORR close to their thermodynamic potential at high rates." | |
| Alireza Kondori, a PhD Candidate in the Asadi Research Group, is first author of a paper in Advanced Materials ("Kinetically Stable Oxide Overlayers on Mo3P Nanoparticles Enabling Lithium–Air Batteries with Low Overpotentials and Long Cycle Life") that reports on a lithium-air battery with long cycle life and low overpotentials. | |
| Generally, to make LAB technology practical, two goals are highly important: a novel cathode that uses a highly active and stable catalysts to enhance ORR and OER kinetics; and a proper electrolyte design that can promote the solvent-based growth mechanism for the discharge products. | |
| In the quest for finding a highly active catalyst, the research team at Illinois Institute of Technology alongside researchers at the University of Pennsylvania, the University of Illinois at Chicago, and Argonne National Laboratory, developed a LAB which benefits from highly active Mo3P nanoparticles with extremely low discharge and charge overpotentials of 80 and 270 mV. | |
| For their LAB, the team used Mo3P (trimolybdenum phosphide) nanoparticles as cathode material for ORR and OER as well as an engineered liquid electrolyte comprising redox mediator additives in an IL-DMSO mixture. | |
| The resulting battery works in ambient air condition with high energy efficiency of 90.2% in the first cycle, an energy density of ∼1500 Wh/kg (about 8 times better than state-of-the-art lithium-ion batteries) and a long cycle life of 1200 full charge/discharge cycles that are world-record values for this technology. | |
 |
|
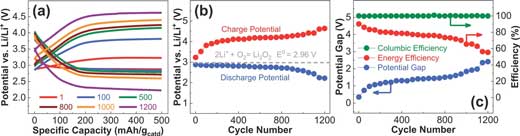
| The Li–air battery performance using Mo3P cathode. a) Charge/discharge profiles over 1200 cycles at a constant density of 500 mA gcatd-1, and a constant specific capacity of 500 mAh gcatd-1. The electrolyte is 0.3 m LiTFSI dissolved in DMSO:EMIM-BF4 (75/25 v/v%) with 25 × 10–3 Μ of each TEMPO and DBBQ RMs. b) Discharge and charge potential values over 1200 cycles. The graph shows the stable discharge overpotential with an average value of 2.77 V over 1000 cycles. c) Changes in columbic efficiency, energy efficiency, and potential gap over 1200 cycles. (Reprinted with permission by Wiley-VCH Verlag) (click on image to enlarge) | |
| To conclude, this work shows that Mo3P nanoparticles are a promising catalyst candidate, owing to their unique structure – a surface providing a high density of molybdenum active sites with special electronic properties, that is, low work function and high density of d-orbital electrons at Fermi energy, which can be promising for electrocatalysis. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
