| Mar 29, 2021 | |
Real-time sweat analysis with wearable microfluidic sensors |
|
| (Nanowerk Spotlight) Smart watches, fitness trackers, electronic textiles, and skin sensor patches are all part of the quickly growing market for personalized healthcare technology. Based on recent advances in wearable electronics, these devices allow for the noninvasive monitoring of real-time physiological health information from a growing number of biomarkers (read more: "How nanotechnology enables wearable electronics"). | |
| Apart from monitoring physiological and kinematic characteristics, epidermal electronic sensors are also suitable for the noninvasive monitoring of chemical species. Of particular interest here is sweat – a rich, chemical concoction containing a number of important chemical compounds with physiological health information. | |
| "The in situ real-time analysis of sweat can avoid the need for repetitive painful blood analysis and other invasive tests," Dr. Vinu Mohan, DST-INSPIRE Faculty & AcSIR Assistant Professor at CSIR-Central Electrochemical Research Institute (CECRI), Karaikudi in India, tells Nanowerk. "An epidermal patch could for instance track the hydration level and oxygenation of muscles, which is essential for fitness monitoring application. These wearable biosensors could be used for clinical diagnosis and personalized point-of-care analysis." | |
| Reporting their findings in ACS Sensors ("Fully Printed Wearable Microfluidic Devices for High-Throughput Sweat Sampling and Multiplexed Electrochemical Analysis"), Mohan and his team have demonstrated the large-scale fabrication of skin-interfaced printed microfluidic patches, capable of multiplexed electrochemical detection of lactate, Na+, K+, and pH from human sweat. | |
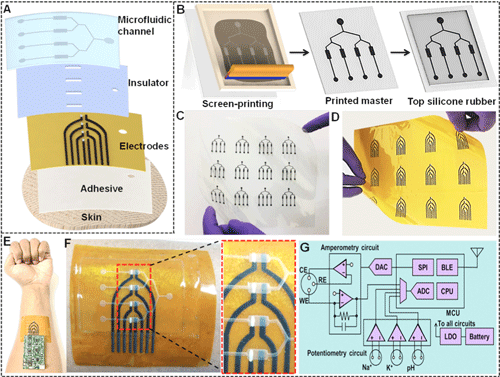 |
|
| Overview of fully printed microfluidic device. (A) Schematic representation of different layers of the microfluidic patch. (B) Schematic illustration of the fabrication of silicone rubber-based microfluidic channels from the screen-printed carbon master. The optical images showing an array of printed (C) carbon master and (D) multiarray electrodes. Images showing (E) the skin-interfaced, fully integrated microfluidic sensor and (F) the close-up part of multiarray electrode having four sensing chambers. (G) Scheme representing the layout of miniature PCB capable of multiplexed analysis and wireless data transmission. (Reprinted with permission from American Chemical Society) (click on image to enlarge) | |
| This sensor continuously and simultaneously measures the level of biomarkers such as lactate, Na+, K+, and pH during sweating. The skin-worn sensor can selectively measure analytes even in the presence of other interfering species present in sweat. | |
| The patch is integrated with a custom-made miniature printed circuit board (PCB) that enables multiplexed decoding of sweat and wireless signal transduction to the host devices and is completely free from any sensor-to-sensor transfer of signals. | |
| "Lactate is a key indicator for pressure ischemia and tissue hypoxia, reflecting the deprived oxygen supply and insufficient oxidative metabolism," Mohan explains. "Sweat lactate level is associated with the mechanism of eccrine gland energy metabolism, and the production of lactate is directly proportional to the exercise intensity. Also, the abnormal level of sweat Na+ is clinically important for diagnosing cystic fibrosis, caused by the failure in Cl- reabsorption at eccrine glands." | |
| One of the key challenges in wearable sweat analysis is the irregular sweat generation and capturing rates, dilution and carry-over effects due to the mixing up of sweat produced at various intervals of time. This means that accurate sweat biomarker analysis requires microfluidic sensors with rapid sweat sampling and multiplexed electrochemical recognition abilities. | |
| "Most of the currently developed microfluidic analytical patches are not suitable for continuous monitoring of sweat and they cannot be re-used," Mohan points out. "They have been developed utilizing conventional photolithographic techniques, which are expensive and time-consuming and hinder large-scale production of electrochemical multianalyte sensing platforms." | |
| In contrast, Mohan's team has introduced a cleanroom-free fabrication of wearable microfluidic sensors based on a low-cost screen-printed carbon master. The sweat sampling is enhanced by introducing low-dimensional sensing compartments and lowering the hydrophilicity of channel layers via facile chemical functionalization. | |
| The fluidic channel captures sweat at the inlet and directs the sweat in real-time via capillary force of attraction – without the need for an external pump – to the active sensing electrodes (within 40 seconds) for subsequent decoding and selective analyses. | |
| As the team points out, their low-cost method allows large-scale fabrication of high-throughput flexible sensors to conduct more statistical analysis including large population studies with different age groups, and to assess the significance of ethnicity. | |
| The current focus is to make the sensor stretchable as well, so that it can maintain its performance even under mechanical deformation. Stretchable sensors can avoid cracking or device damage during irregular body motion or muscle movements. | |
| Another complexity lies in the fact that the nature of sweat production varies from person to person where gender and ethnicity also play a major role. In addition, non-uniform sweat generation at different parts of the body also creates hurdles for precise sweat monitoring. Frequent calibration processes and temperature based corrections are also important for accurate biomarkers tracking. | |
| "Going forward, our microfluidic sensor offers the flexibility to exploit or reconfigure for real-time quantification of other relevant biomarkers that could be detected from sweat and other biofluids," Mohan concludes. "Large data sets could be collected via numerous population studies based on gender and ethnicity that would enable us to generate precise predictive algorithms for effective commercialization processes and to monitor the real-time health status of individuals for personalized point-of-care analysis." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
