| Apr 19, 2021 | |
Enhancing plasmonic biosensors with atomically thin nanomaterials |
|
| (Nanowerk Spotlight) Plasmonic sensors or surface plasmon resonance (SPR) sensors are some of the most commonly-used optical sensing devices for real-time monitoring of chemical and biomolecular interactions. | |
| SPR is very sensitive to the surrounding environment, a property that is utilized in real-time and label-free detections. SPR sensors have been commercialized for more two decades, and they represent the current 'gold standard' for label-free biosensing. These sensors have been applied in various areas including food quality control, environmental monitoring, drug screening, and early-stage disease diagnosis. | |
| But there are limits to SPR-based sensing, which makes it unsuitable for sensing small target analytes with a molecular weight less than 400 Dalton; especially for cancer biomarkers, antibiotics, thyroid hormones, peptides, steroids, and bacterial pathogens in infectious diseases. | |
| Also, SPR's detection limit is not good enough to find biological and chemical molecules with low concentration levels (i.e., < 10-15 mol/L) in complex matrices such as urine, saliva, and blood serum. | |
| This ability to detect specific biomarkers at extremely low concentration levels, however, is critical to early-stage diagnostics of a variety of human diseases. | |
| Among the various biomarkers, tumor necrosis factor (TNF-α) is a widely accepted biomarker for a variety of human diseases, including inflammatory disorder such as bowel disease, osteoarthritis, rheumatoid arthritis as well as malignant tumors such as oral and breast cancer. | |
| Unfortunately, the minute amounts of this protein circulating in blood makes detecting the molecule and measuring its concentration accurately a technological challenge. | |
| "The concentration levels of biomarkers in general, and TNF-α in particular, is generally extremely low, typically ∼20 pg/mL in a healthy human," Dr. Shuwen Zeng from the XLIM Research Institute at the Université de Limoges, tells Nanowerk. "In addition, the molecular weight of TNF-α (∼17 kDa) is one order of magnitude lower than many common biomarkers, further complicating efforts to detect it in blood samples." | |
| Conventional TNF-α detection techniques include enzyme-linked immunosorbent assay (ELISA), immuno-PCR and fluorescence assay. However, these detection methods can be time-consuming and often require complex operations with adequate transducing elements such as fluorescent dyes or enzymes. | |
| This has led researchers to develop techniques related to detection of this biomarker at low cost and with ultrahigh sensitivity. | |
| Recently, a group of researchers, including Zeng, have significantly enhanced the performance of a SPR biosensing platform by adding an atomically thin phase change material to induce a giant lateral position shift called Goos–Hänchen (GH) shift, which in turn leads to the detection of TNF-α cancer biomarkers at sub-attomole level. | |
| They report their findings in Nano-Micro Letters ("Targeted Sub-Attomole Cancer Biomarker Detection Based on Phase Singularity 2D Nanomaterial-Enhanced Plasmonic Biosensor") | |
| "We tuned two-dimensional Ge2Sb2Te5 (GST) nanomaterials, which have a high absorption rate in visible and near-infrared wavelengths, to achieve zero-reflection at plasmon resonance," Zeng explains. "The zero-reflection phenomenon resulted in a strong phase singularity." | |
| "This zero-reflection induced phase singularity is known to be challenging to achieve in previous plasmonic nanostructures," he adds. "We found the higher order mode of the phase signal, i.e. the lateral position shift, to be much larger than other signal modalities reported in recent years." | |
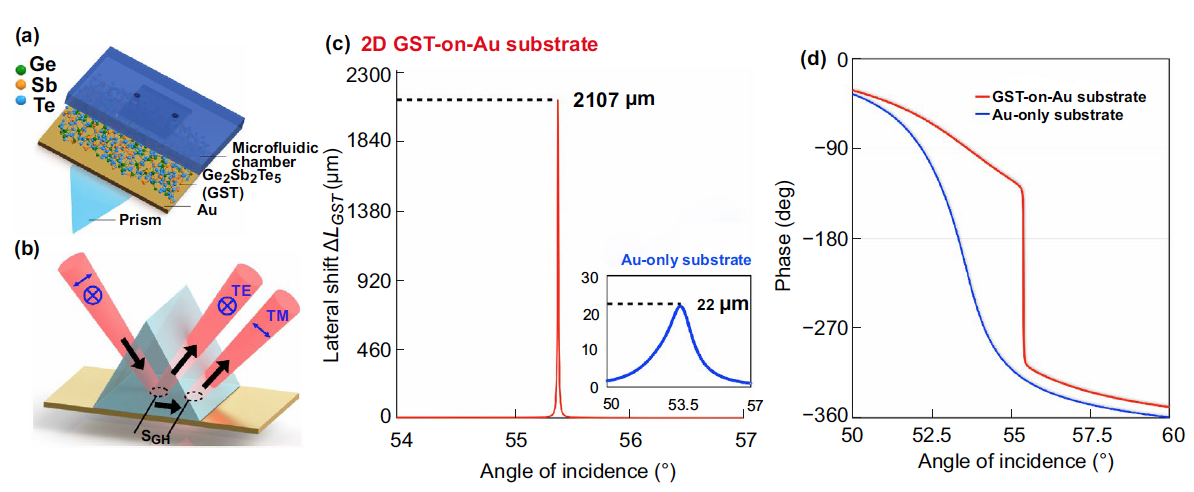 |
|
| (a) Schematic of the sensing substrate based on GST-gold metastructures. (b) Schematic diagram of the giant lateral position shift induced by the GST-on-Au substrate. Simulation results of (c) lateral position shift and (d) optical phase signal change based on 2D GST-on-Au substrate and Au-only substrate. (Reprinted with permission by Springer Nature under a Creative Commons CC BY license) | |
| The plasmonic sensing device that the team developed exhibits a detection limit of 10-15 mol/L (1 fM) for TNF-α biomarkers (which is more than three orders of magnitude compared to current state-of-art nanomaterial-enhanced plasmonic sensors) and 10-14 mol/L (10 fM) for small biotin molecules (MW=244.31 Da). | |
| "This sub-attomole detection level is a significant improvement compared to other SPR designs," Zeng points out. "The maximum experimental lateral position shift triggered in our device is 341.90 µm, which to our best knowledge is the largest value ever reported." | |
| In the next stage of their research, the team plans to further optimize the surface functionalization scheme to improve the specificity of their device. Moreover, they want to integrate microfluidic chips with plasmonic sensors for multiplexed detection of various kinds of cancer biomarkers at the same time. | |
| "The research topic of 2D nanomaterials-based plasmonic sensors is still in the infant stage," Zeng concludes. "One challenge is that the high absorption rate of GST phase change materials only exists in visible and near-infrared regions. More materials should be explored in other detection wavelength. Also, the optimization of the sensing substrate with different 2D materials should be further studied to achieve better sensing performance." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
