| Posted: Jun 23, 2006 | |
Understanding the growth mechanisms of nanowires |
|
| (Nanowerk Spotlight) One-dimensional nanostructures such as nanowires, in particular semiconductor nanowires, have unique applications in the fabrication of nanoscale devices. How to control the growth of semiconductor nanowires is one of the most challenging issues presently faced by synthesis chemists. | |
| In a recent paper, titled "Solvothermal/hydrothermal route to semiconductor nanowires" researchers at the University of Science and Technology of China in Hefei, PR China, present a brief summary about nanowires synthesis and growth in solvothermal/hydrothermal process. | |
| "In the past decade, significant progress in nanowire growth has been made, in terms of improved uniformity, fine monodispersion, and high yield of product. Nanowires have been grown through many methods, such as electrochemistry, templates (mesoporous silica, carbon nanotubes, etc), emulsion or polymeric systems, arc discharge and laser-assisted catalytic growth, solution, and vapor transport methods. Despite these exciting developments, a general synthetic strategy to grow nanowires is still significant to both nanotechnology and fundamental research" Professor Yitai Qian explains the background of the recent paper to Nanowerk. | |
| In their recent work, the Chinese researchers explore how solvothermal (using non-water as solvents) and hydrothermal methods can be used to control the synthesis of semiconductor nanowires. They demonstrated that four different aspects have an important effect on these methods: | |
| Materials with highly anisotropic crystal structures - many solid materials easily grow into 1D nanowires, and this phenomenon is determined by the highly anisotropic bonding in the crystallographic structure. Usually the the crystallization tends to occur along a certain axis. | |
| Coordination directing/mixed solvents - the solvents used in solvothermal/hydrothermal synthesis have proved to have key effects in the formation of semiconductor nanowires, where they serve as the structure-directing coordination (template effect) during nanowires formation. | |
| Surfactants/capping reagents - The shapes of nanocrystals are determined by the relatively specific surface energies associated with facets of the crystal. One can control the final shape of a crystal by introducing appropriate surfactants/capping reagents to change the free energies of the various crystallographic surfaces and thus to alter their growth rates. | |
| Reaction at relatively high temperature - the vapor–liquid–solid (VLS) process has been successfully utilized to produce semiconductor nanowires. This method generally needs expensive apparatus, high reaction temperature, and complex procedures. In future work, the solvothermal process may replace some VLS work in the synthesis of semiconductor nanowires. | |
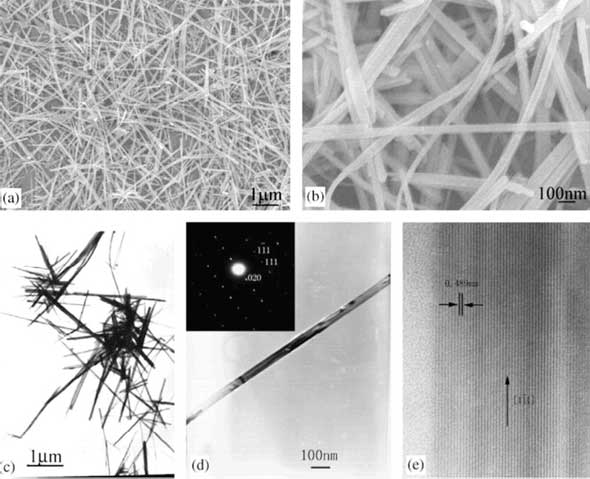 | |
| (a), (b) FESEM images of single-crystalline α-MnO2 nanowires: (a) lower magnification; (b) higher magnification; (c) TEM image of the nanowires; (d) typical TEM image of the nanowires; the inset is the SAED pattern; (e) HRTEM image of the nanowire in (d). (Reprinted with permission from IoP Publishing) | |
| "These may provide wider and significant considerations for future synthesis of nanowires and investigation of their growth mechanism" says Qian. "With greater understanding of nanowire growth mechanisms, we think that more nanowires of various materials and with novel shapes may be obtained through proper change of reaction conditions in solvothermal/hydrothermal synthesis." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
