| Posted: Jun 05, 2008 | |
Military nanotechnology: high precision explosives through nanoscale structuring |
|
| (Nanowerk Spotlight) Usually, when your read official government publications about the military's nanotechnology research and development activities, it's all about sensors, batteries, wound care, filtration systems, smart fabrics, and lighter, stronger, heat-resistant nanocomposite materials etc. It's all quite useful stuff for non-military applications as well, and - as described by these sources - it's all just for defensive purposes. | |
| A good example is the official and public annual report by the U.S. Department of Defense "Defence Nanotechnology Research and Development Program". Official sources keep quite mum though about military research into offensive nanotechnology applications. For instance, in the above-mentioned DoD report the words "explosive", "ammunition" or "bomb" don't appear even once. Does that mean the military is not researching nanotechnology applications for more effective ways of blowing stuff up, or are they just being tight-lipped about it? Your guess... | |
| Of course there is plenty of potential for offensive military nanotechnology applications. As we have reported before, a NATO study group has stated that "the potential for nanotechnology-driven innovations in chemical and biological weapons are particularly disquieting as they can considerably enhance the delivery mechanisms of agents or toxic substances. The ability of nanoparticles to penetrate the human body and its cells could make biological and chemical warfare much more feasible, easier to manage and to direct against specific groups or individuals." | |
| Case in point of how nanotechnology could be used for offensive military applications can be found in recent studies exploring how high explosive materials can be prepared and manipulated. Engineering and control of energetic material (another, more innocent sounding term for 'explosives') properties at the nanoscale are of paramount importance when the ignition and detonation properties of high explosives are to be determined. | |
| Last year for instance, researchers presented methods for making continuous high explosives thin films and arbitrary high explosives patterns at the nanoscale ("Patterning High Explosives at the Nanoscale"). | |
| Another, more recent example is a study by French scientists who report the first attempt to control the combustion and the detonation properties of a high explosive through its structure. | |
| Writing in the journal Nanotechnology, researchers from the Laboratoire ISL/CNRS 'Nanomatériaux pour les Systèmes Sous Sollicitations Extrêmes' at the French–German Research Institute of Saint-Louis in France describe that they have prepared energetic nanocomposites with different formulations by infiltrating a porous chromium oxide matrix with a high explosive dissolved in acetone ("Preparation of explosive nanoparticles in a porous chromium(III) oxide matrix: a first attempt to control the reactivity of explosives"). The scientists claim that their method allows one to obtain and stabilize high explosive particles at the nanoscale. | |
| The ISL scientists write that, until now, the only way to tune the explosive reactivity was to mix several chemicals in order to obtain a composition with the right properties. The idea reported in this paper – adjusting the reactive properties through the structure of the explosive – appears much neater. | |
| Two of the paper's authors, Marc Comet and Denis Spitzer, who both work at the French-German Research Institute (ISL - Research for Security and Defence), have previously described a theoretical model that shows that a significant change in the reactive properties of high explosives is expected for particles with a size between 10 and 30 nm (Des thermites classiques aux composites interstitiels métastables). | |
| "From an experimental standpoint, the main problem arises from the stabilization of the explosive nanoparticles at the nanoscale" the ISL team says. "For this purpose, one of the most promising solutions is to trap explosive particles in the open porosity of an inert matrix." | |
| In their experiments, the porous matrix used was chromium (III) oxide. This inorganic matrix was used as a template to enclose and stabilize explosive nanoparticles, in this case hexogen (RDX), an explosive nitroamine widely used in military applications. As a result, the reactive behavior of explosive nanoparticles trapped in the inorganic matrix is defined by their size and their distribution in the chromium oxide. | |
 |
|
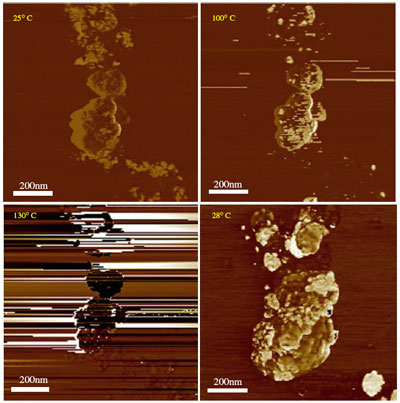
| AFM images of the thermal evolution of a Cr2O3/RDX nanocomposite particle (40.0 wt% RDX). (Reprinted with permission from IOP Publishing) | |
| The ISL team confirmed the decomposition mechanism of their nanocomposite explosive by atomic force microscopy (AFM) in tapping mode. They describe this in their paper: "A nanocomposite particle (40 wt% RDX) was first imaged at 25 °C in order to visualize its original appearance. Afterwards, the sample was heated to 100 °C, where the initial decomposition phenomena were noticed in the phase signal. These events are characterized by perturbation lines coming from the smallest particles. Further heating to 130 °C enhances the decomposition of the material. After being heated to 250 °C, the material was cooled down to room temperature (28 °C). It exhibits a cauliflower structure with a strong spatial expansion of about two times compared to the initial structure." | |
| This is the first time that a nano explosion (of course the authors describe this in more scientific terms as "the incidence of the decomposition of an explosive at the nanoscale") has been imaged in such detail. | |
| Because the stabilization of high explosives by porous materials allows controlling their reactivity, practical applications of this research will make it possible to design energetic materials according to precise needs. The ISL scientists say that, for instance, the formulation of gun powders or propellants can be adjusted so as to avoid detonation and to define the combustion rate. Conversely, this process can be used to tune the detonation velocity of high explosives. | |
| More bang for the buck, so to speak. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
