| May 11, 2022 | |
Graphdiyne functionalized by silver nanoparticles to combat the threat of antibacterial resistance |
|
| (Nanowerk Spotlight) The threat of resistance to antimicrobial agents has been growing at an alarming rate in recent years and poses a huge public health threat globally according to the WHO. The increase in morbidity and mortality resulting from microbial infections has been attributed to the emergence of multidrug-resistant microbes. | |
| According to the CDC, in the U.S. alone, this causes more than 2 million infections and 23,000 deaths per year. Worldwide, antibiotic resistance threatens our progress in healthcare, food production, and ultimately life expectancy. | |
| Associated with the increase in multidrug resistance is the lack of new and effective antibacterial drugs. This has led to global initiatives to identify novel and more effective antimicrobial agents in addition to discovering novel and effective drug delivery and targeting methods. | |
| The use of nanoparticles as novel biomaterials to fully overcome this challenge is currently gaining global attention. Nanoparticles could become an indispensable viable therapeutic option for treating drug-resistant infections. | |
| Silver is well-known as the most universal antimicrobial substance due to its strong biocidal effect against microorganisms. Silver nanoparticles (AgNPs) can continually release silver ions, which may be considered the mechanism of killing microbes. | |
| Owing to electrostatic attraction and affinity to sulfur proteins, silver ions can adhere to the cell wall and cytoplasmic membrane (a biological membrane that separates the interior of a cell from its outside). The adhered ions can enhance the permeability of the cytoplasmic membrane and lead to disruption of the bacterial envelope, in effect destroying the bacteria. | |
| Although bacterial resistance to antibiotics has been extensively discussed in the literature, the possible development of resistance to silver nanoparticles has not been fully explored. For instance, a recent study reports that the Gram-negative bacteria Escherichia coli 013, Pseudomonas aeruginosa CCM 3955 and E. coli CCM 3954 can develop resistance to silver nanoparticles after repeated exposure. | |
| The resistance arises from the production of the adhesive flagellum protein flagellin, which triggers the aggregation of the nanoparticles. This resistance evolves without any genetic changes; only phenotypic change is needed to reduce the nanoparticles’ colloidal stability and thus eliminate their antibacterial activity. | |
| The resistance mechanism cannot be overcome by additional stabilization of silver nanoparticles using surfactants or polymers. It is, however, strongly suppressed by inhibiting flagellin production with pomegranate rind extract. | |
| Moreover, silver nanoparticles have aninherent tendency to agglomerate in media due to their larger surface-to-volume ratio and higher surface energy. | |
| In view of the above mentioned facts, it is of crucial importance to control the stability of AgNPs in order to maintain their excellent antibacterial activity for practical applications. Significant efforts have been made to stabilize silver nanoparticles (i.e., avoiding their agglomeration) by employing an anchor for its growth, such as polymers, silicon-based materials and carbon-based materials. | |
| Among them, AgNPs decorated graphene oxide (GO) nanocomposite (GO-Ag) prevents the tendency to agglomerate and displays very low cytotoxicity and shows highly effective antibacterial activities against E. coli and S. aureus. | |
| Due to its unique sp–sp2 carbon atoms, uniform pores, and highly π-conjugated structure, the two-dimensional carbon allotrope Graphdiyne (GDY) offers promising potential in practical applications, such as gas separation, catalysis, water remediation, humidity sensor, and energy-related fields and biomedicals. | |
| Graphdiyne's well-distributed triangular pores exhibit higher affinity with molecules, ions and compounds. Especially, the plane π/π* states strengthen the combination between metal atoms and alkynyl, making GDY an ideal substrate for metal nanoparticles. | |
| Various GDY metal or metal oxide nanocomposites have been reported, for example, Ni/graphdiyne and Fe/graphdiyne, palladium-iron nanostructure-coated graphdiyne nanosheet (PdFe/GDY) and TiO2/graphdiyne composites (TiO2/GDY). | |
| The antibacterial activity of graphdiyne and graphdiyne oxide (GDYO) has already been reported. GDY is capable of inhibiting broad-spectrum bacterial growth while exerting moderate cytotoxicity on mammalian cells. In comparison, GDYO exhibits lower antibacterial activity than that of GDY. The antibacterial activity of GDY may be contributed to a synergistic mechanism of wrapping, insertion, disruption, and oxidative stress on bacterial membrane. | |
| In recent work, researchers demonstrate a high-performance bactericid with graphdiyne functionalized by silver nanoparticles (GDY@Ag). GDY@Ag was synthesized by simply mixing silver nitrate with GDY and a surfactant was added to assist the growth of nanoparticles. The synthesis was carried out by an environment-friendly approach without any reductants. In this study, Bacillus subtilis and E. coli were selected to represent gram-positive and gram-negative bacteria, respectively. | |
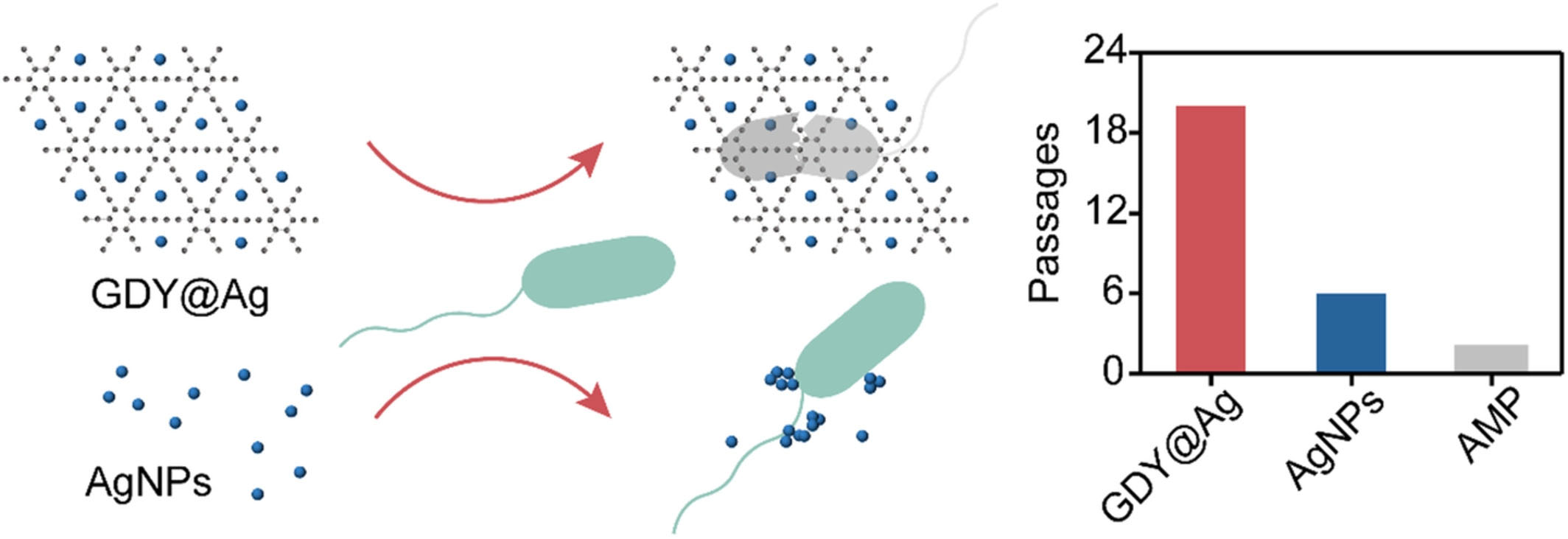
| The acetylenic groups in GDY acted not only as a reductant, but also as an anchor for the growth of AgNPs. The hybrid GDY@Ag showed exceptional broad-spectrum antibacterial activity towards both gram-positive and gram-negative bacteria. The scientists found that GDY@Ag killed the bacteria through membrane destruction and reactive oxygen species (ROS) production. Moreover, neither bacterial strain developed a resistance to GDY@Ag after repeated exposure (Figure 1). | |
| The researchers concluded that the antibacterial mechanism of GDY@Ag may be contributed to a synergistic action of GDY and AgNPs. GDY@Ag first wrapped bacteria through electrostatic interaction, and then 'insertion mode' and oxidative stress combined to destroy the cell membrane, ultimately resulting in bacterial death. | |
| These findings present an avenue to fabricate new antibacterial agents for effective bactericidal activity. | |
 |
|
| Figure 1. Schematic of stable GDY@Ag with increased antimicrobial property and bacterial susceptibility compared to unstable AgNPs and (AMP: Ampicillin). (Reprinted from doi:10.1111/cpr.13236 with permission by John Wiley and Sons under Creative Commons Attribution 4.0 International (CC BY 4.0)) | |
| Based on earlier studies, there is a strong possibility that both GDY and graphdiyne oxide (GDYO) and their metal nanoparticle functionalized forms are capable of hindering broad-spectrum bacterial growth. Therefore, the authors postulate that silver nanoparticle functionalized GDY may not only increase the stability of silver nanoparticles, but also provide a new bactericide with a synergistic antimicrobial effect. They also suggest that graphdiyne should be combined with other effective bactericides to achieve greater synergistic antisepsis. | |
| Notwithstanding the unexpected emergence of metal nanoparticle associated antimicrobial resistance, the present study has now opened the door to harnessing 2D materials to stabilize metal nanoparticles as a promising option for combating evolving bacteria. | |
|
By Yashwant Mahajan, Associate Editor, Nanowerk
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
