| Jun 03, 2022 | |
'Self-aware' and self-powered metamaterial implants |
|
| (Nanowerk Spotlight) Medical research is moving quickly towards a future where smart medical implants can continuously monitor their condition inside the body and autonomously respond to changes such as for instance infection by releasing anti-inflammatory agents. Some examples that researchers have already demonstrated are sensors that can be placed under the skin for measuring blood glucose levels, hormone levels, pH and various other physical parameters; the use of polypyrrole films as electrically controlled drug release devices on implant surfaces to improve bone implants; electronic under-the-skin sensors to monitor blood flow; and pressure sensors to improve medical implants. | |
| Today, intrinsic diagnostic functionality is the missing component of almost all available implants. Where smart implants have been demonstrated, they often rely on power sources such as batteries, capacitors or external telemetry systems. | |
| Currently there are two major challenges that constrain the wider application of smart implants in daily clinical practice. The first challenge is the size of these implants for sensor integration. For example, imagine the difficulties associated with integrating multiple circuit boards for sensing, energy storage and wireless communications into the small area of a miniaturized stent. The second challenge is the lack of scalable and smart biomaterials for fabricating these devices. | |
| There have been some studies trying to address these issues separately. But so far, no research team has tackled all these issues comprehensively by designing a multifunctional implant that can diagnose the healing progress, can power itself, and can be tuned to offer almost any desired mechanical performance. | |
| A potential solution to all of these challenges is to use the implant matrix as an active sensing and energy harvesting medium. Doing so removes the need to figure out how to integrate bulky circuits or power sources into the small device area of an implant. | |
| Writing in Advanced Functional Materials ("Patient-Specific Self-Powered Metamaterial Implants for Detecting Bone Healing Progress"), researchers now propose the first-of-a-kind smart orthopedic implant with both diagnostic and energy harvesting capabilities. | |
| This mechanically tunable, multifunctional metamaterial implant can sense and harvest energy from body motions. | |
| "Our concept is clinically significant because these implants enable surgeons to directly and precisely assess the bone healing progress," Amir H. Alavi, an Assistant Professor in the Department of Bioengineering at the University of Pittsburgh, tells Nanowerk. "These features can potentially eliminate the need for radiographic imaging techniques, which are usually costly and expose patients to significant radiation." | |
| In what could be a game-changer, these implants only use their constituent components to achieve these advanced functionalities – they don't require any external power source or bulky electronics. Furthermore, the implants can be 3D printed and customized for each patient based on the clinical requirements and anatomical matching. | |
| "We rely on the rational design of the implants' microlayers to embed advanced functionalities into their matrix," explains Alavi. "Think about the fact that you can use our technology to transform literally any implantable device into a sensor and nanogenerator by manipulating their geometrical designs. There are tons of biocompatible material options that can be used under this technology." | |
| This fabrication technique is mainly based on the team's patented meta-tribomaterial technology. This technology deals with rational design of multiple layers of triboelectric auxetic microstructures with multi-stable/self-recovering snapping segments. | |
| The beauty of this concept is that the same design works at both nanoscale and macroscale simply by tailoring the design geometry. | |
| Building on their recent recent study on meta-tribomaterial sensor and nanogenerators ("Self-aware materials build the foundation for living structures"), the team uses different rationally-designed triboelectric auxetic microstructures to build the implant. | |
| The entire implant structure serves as an energy harvesting medium as well as an active sensing system. | |
 |
|
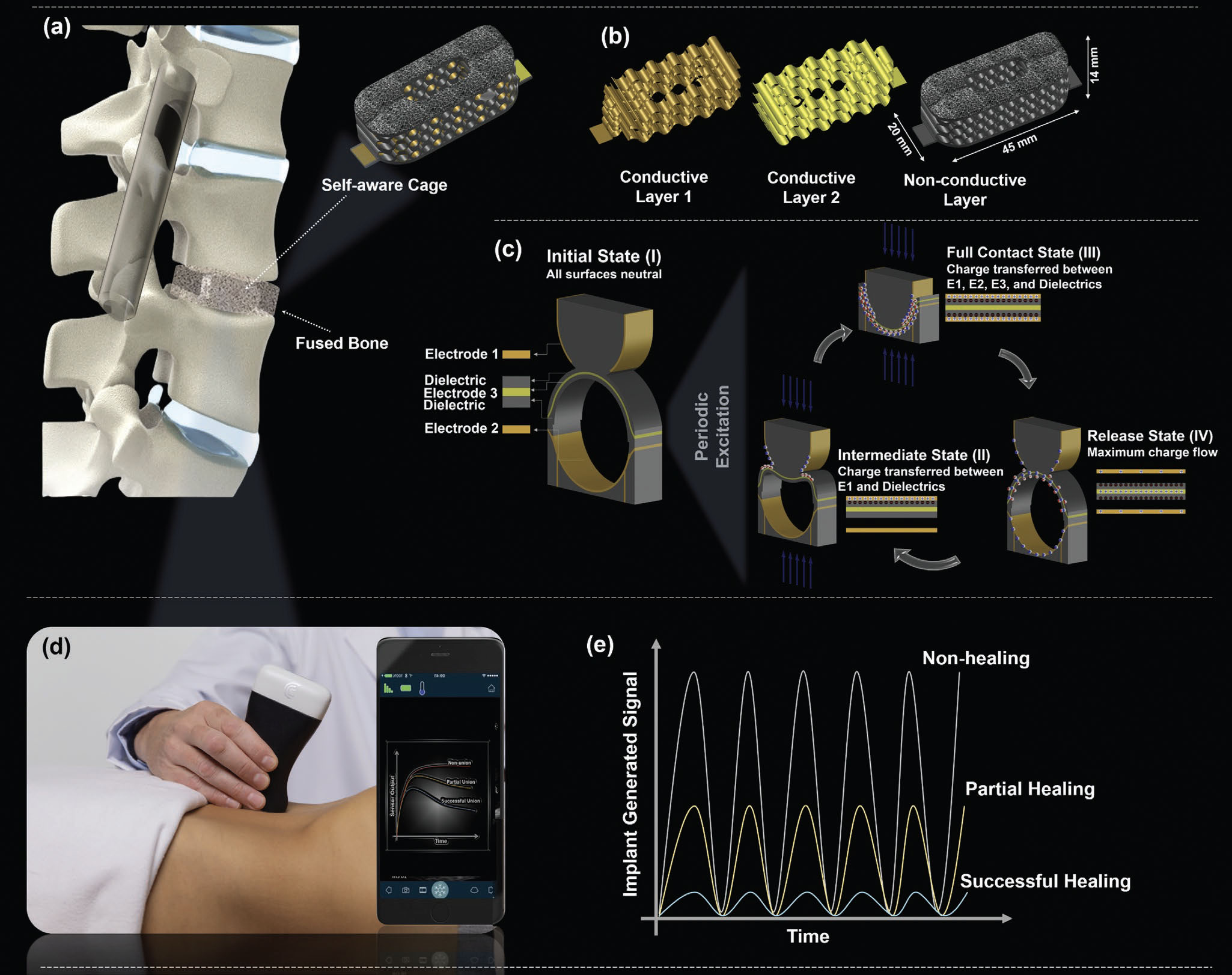
| Vision of the proposed research showing a self-aware metamaterial implant that can be used for reliable determination of spinal fusion development post-surgery directly at the intervertebral level. a) A multifunctional nanogenerator interbody fusion cage with self-recovering, self-sensing and energy harvesting functionalities implanted during spinal fusion surgery. b) Composition of a self-aware cage implant. The implant generates electrical signals due to spine micro-motions using its built-in contact-electrification mechanism. The signal can be used for sensing and energy harvesting purposes. c) Physics mechanisms of the built-in contact-electrification in self-aware implants. d) The recorded data will be retrieved using an FDA-compliant portable ultrasound scanner. This figure shows a Clarius C3 HD3 ultrasound scanner. e) The sensor output signals represent various healing stages and can be correlated with the changes of FSU stiffness due to the healing process. (reprinted with permission from Wiley-VCH Verlag) (click on image to enlarge) | |
| According to the team, orthopedic implants seem to be the most immediate application area for this technology since the implants require mechanical vibrations to harvest energy for self-powering. | |
| Consequently, they highlight the features and underlying mechanisms of their proposed technology by creating a proof-of-concept spinal fusion cage prototype that harvests energy from the micro-motions of the spine. The generated electrical signal is then used for diagnostic purposes. | |
| As illustrated in the figure above, the self-aware metamaterial fusion cage can detect various levels of spinal fusion through continuous stability and load-sharing measurements directly at the intervertebral disc space level. These features provide physicians the ability to assess the progress of fusion without the need for radiographic imaging. | |
| Other medical fields can also benefit from this technology. For instance, the same approach could be used to design smart cardiac stents with sensing and energy harvesting functionalities. | |
 |
|
| Close-up of a multifunctional nanogenerator interbody fusion cage with self-recovering, self-sensing and energy harvesting functionalities. (Image courtesy of the researchers) | |
| The researchers have already tested these spinal implants using synthetic spine and human cadaver spine models. Their next step is to study their performance in vivo using large animal models to establish a preclinical basis prior to a human clinical trial. | |
| "Under loading conditions similar to human lumbar spine, our fusion cage prototype can generate voltage and current values equal to 9.2 V and 4.9 nA, respectively," says Alavi. "A series of fatigue tests using the synthetic spine model revealed that the cage elastic modulus drops from 1.76 to 1.4 MPa after 40 000 loading cycles. The results imply the necessity to develop more robust fabrication and calibration methods for the long-term performance of the implants in vivo." | |
| Currently, the main challenge is the wireless interrogation of the data measured by the implants and the team is exploring viable solutions to deal with this issue. For example, they have been closely working with their collaborators at Washington University to couple the electrical signals generated by the self-aware implants with ultra-low-power wireless data logging technologies to create fully self-powered systems. Of course, the implant interrogation can also be readily performed via simpler passive strategies such as existing RFID telemetry systems. | |
 |
|
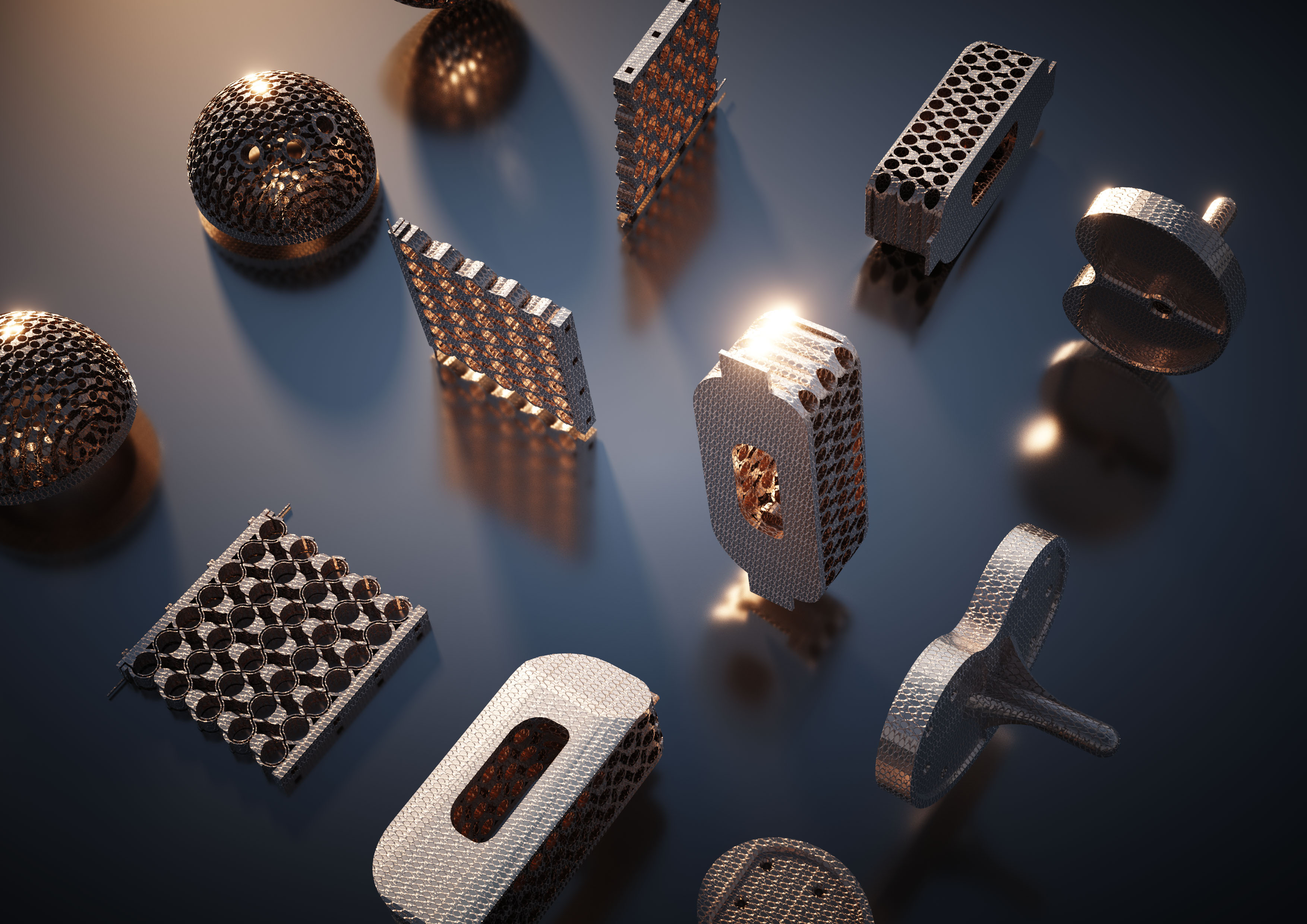
| The team demonstrated a wide range of patient- and use-specific fusion cage implant prototypes. (Image courtesy of the researchers) | |
| Over time, the researchers envision to create 3D nano-, micro-, meso-, and macro-scale implants with their technology. | |
| "We are confident our concept can push the limits of many of the existing medical implant technologies," Alavi concludes. "The reason is that it enables us creating implants that can perform sensing, energy harvesting, and information processing using their own matrix." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
