| Jun 10, 2022 | |
Researchers develop 'attacking' droplets as a step towards biomimetic nanosystems (w/video) |
|
| (Nanowerk Spotlight) One of the key goals of nanochemistry is to develop artificial systems that imitate biological processes, particularly aiming at replicating the basic behavior of life forms. In this field, the interaction between droplets is gaining increasing attention as model systems to approach the dynamic behaviors of living cells, which have evolved to harvest energy from their environments to drive the chemical processes that enable life. | |
| The development of these 'droplet frameworks' is an essential tool to understand, explain and eventually replicate Nature's processes during the early steps in the origin of life on Earth. One hypothesis is that small droplets could have formed via the segregation of molecules from complex mixtures by phase separation. These droplets could have provided chemical reaction centers, ultimately leading to the evolutionary transition path from inanimate chemical to living biological systems. | |
| Researchers have now demonstrated that basic features found in many living cell types can emerge from a simple two-droplet framework. In Nature Communications ("Droplets in underlying chemical communication recreate cell interaction behaviors") a team of scientists from four institutions in Argentina (CONICET, CNEA and the Universities of San Martín and Litoral) reports that a pair of droplets loaded with chemical reagents deposited on a nanoporous surface can communicate in a somewhat intelligent form, presenting a chemospecific stimulus-response autonomous behavior. | |
| "We demonstrate that two droplets deposited on a nanoporous surface are able to communicate through chemical signals that travel across the nanopore network," Dr. Galo Soler-Illia from the Universidad Nacional de San Martín, tells Nanowerk. "This communication results in a biomimetic behavior, as one of the droplets can move towards the other one, displaying an 'attacker-victim' action." | |
| In the research of droplet behavior on surfaces, previous works by various groups have focused on aspects related to wettability, adhesion and transport across nanopore surfaces. This new work, though, raises a unique and exciting concept: coding a behavior of an in-principle inanimate object by choosing a suitable chemical process to fuel an irreversible transformation, and by providing channels for the adequate funneling of energy and chemical information, in order to transduce a local process into a macroscopic movement that implies an organized displacement of soft matter. | |
| The most exciting feature of this work, to put it differently, is the possibility of creating active droplets that interact and act by themselves via messages through a surface. The team shows that a specific vectorial action – the movement towards a second object – can be imparted to an inanimate object through a simple platform that involves chemosensing. In other words, it is possible to 'program' the behavior of an inanimate macroscopic object in a way that resembles a cellular response. | |
| "This attacker-victim reaction between two spatially separated droplets is fueled by a chemical reaction and is crucially dependent on the tuning of nanoscale processes: a fast catalytic reaction in a confined volume, and the transmission of free energy and chemical signals through the nanopores," explains Soler-Illia. "Interestingly, these nano-enabled processes channel enough energy and power to produce significant mechanical changes at the millimeter scale in a short time frame that leads to the observed movement." | |
 |
|
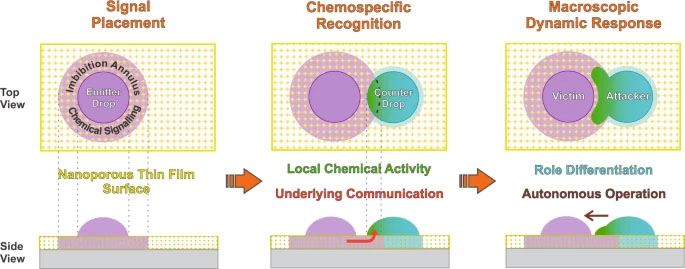
| Schematic concept of the underlying nanoporous layer mediation to achieve complex inter-droplet responses. When chemically complementary drops are deposited together onto a nanoporous thin film, they interact and subsequently act by themselves via underlying chemical messages and localized activity, leading to a macroscopic response. These droplets can spontaneously evolve to a chemospecific stimulus-response operation, which resembles an emergent 'intelligent' behavior. The triggered autonomous attacker-victim-like non-reciprocal interactions lead to the generation of distinctive droplet dynamics with shape-transformation and complex behaviors. Note that the schemes are out of scale (e.g., the thickness of the nanoporous thin film is about a few hundred nanometers, which is much smaller than the droplet height). (Image reproduced from Nature Communications under CC BY 4.0) | |
| As illustrated in the figure above, first, a droplet containing potassium iodide (KI) is deposited on the nanoporous surface. Capillary forces pull the KI solution inside the nanopores, leading to a wet region around the droplet. Then, a second droplet loaded with hydrogen peroxide is placed near the first one. Capillarity works its magic, and these two droplets make contact in a well-defined and confined region of space. A chemical reaction ensues in which KI acts as a catalyst for peroxide decomposition. | |
| The energy released in this reaction, in the confined space of several hundred cubic nanometers, triggers a macroscopic morphological shift in the second droplet – and ultimately the coupling with the neighboring emitter droplet – making use of the fluid released from the counter-droplet across the nanoporous surface as both signaling and power source. | |
| "After exploring several reaction conditions, we found a regime in which the peroxide drop dramatically changes its shape, reaches over the KI-containing droplet and engulfs it," Soler-Illia describes the investigation. "Key to this behavior is the self-propelling expansion of the reaction front along the KI-wet region. In other words, the KI acts like an 'enabling signal' to the reaction to proceed across the surface, and the KI-wet zone acts like a pathway to the 'attacking' droplet." | |
| When a droplet of 30 wt% hydrogen peroxide solution is placed near the KI drop, a behavior ensues, which can be figuratively envisaged as a droplet attacking after the perception of the presence of the victim drop. The H2O2 droplet initially spreads with the typical circular shape until it reaches equilibrium. A distinctive behavior occurs when the drop 'notices' the KI droplet in the vicinity and then extends a protrusion as a leading edge. The H2O2 droplet indeed takes action in response to the fluid released to the nanopores by neighboring KI droplet that self-generate a surrounding attractor region. The protrusion then grows from the H2O2 drop and its size increases gradually up to meet the sessile KI droplet, eventually bridging the droplets. (Video courtesy of the researchers) | |
| He also points out that it is the efficient channeling of the Gibbs energy to breaking the droplet surface tension that leads to the observed shape disruption as the main physical ingredient behind the observed behavior. | |
| Consequently, the team developed a model that permits them to rationalize this cell-like behavior. They demonstrated that the free energy released in the first instants of the peroxide decomposition is more than enough to fuel the rupture of the pinning line of the peroxide droplet, leading to its shape instability. Subsequently, this leads to a gradient of surface tension that eventually triggers a Marangoni effect. This permits the drop to extend on the surface, reaching the larger KI volume. This phenomenon, in turn, creates a positive feedback loop that makes the whole process more irreversible and leads to an even faster drop extension. | |
| In the long term, this kind of process in which a subtle, local transformation is amplified over several orders of magnitude lengths is interesting for the design processes for controlled autonomous movement of nano-objects (nanoswimmers) or the macroscopic movement of soft matter, such as artificial muscles for soft robotics. | |
| As the researchers point out, this very simple system in itself is a minimum unit for a complex signal-motion behavior. It could impact a number of blossoming fields such as the development of responsive micro- and nanofluidics systems, adaptive materials and surfaces, the design of microfluidic smart robotics, or even as a model for biological processes including synthetic biology and origin-of-life protocell models. | |
| In their next steps, the team is planning to integrate these nanoporous systems into stimulable systems, aiming at coupling the transport, chemical reactivity and macroscopic movement already developed with external stimuli such as light, heat, chemical concentration, pH or stress. | |
| "The most relevant challenge in the field of responsive and adaptive materials is to understand the pathways that permit to communicate and transduce signals within nanosystems, and evolve to autonomous, programmable and stimulus-responsive matter," Soler-Illia concludes. "We believe that our work is a step towards a third generation of nanosystems that aim at replicating life processes." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
