| Jun 20, 2022 | |
Learning to understand how nanomaterials enter cells and how cells react |
|
| (Nanowerk Spotlight) The formation of supramolecular complexes between DNA and carbon nanotubes (CNTs) – both single-walled and multi-walled – has drawn significant attention from researchers ever since the first DNA-wrapped CNTs were synthesized in 2003. These hybrid structures can take advantage of the unique mechanical, electrical, thermal, and optical properties of the nanotubes and combine them with the remarkable bio-recognition capabilities of DNA. DNA-CNT supramolecular complexes have been proposed for applications including chemical biosensors, cellular transporters that target structures like mRNA inside cells, fibers for artificial muscles, and bioelectrodes for fuel cells. | |
| Although scientists know that CNTs can penetrate pretty much every cell (-wall), the detailed mechanism of uptake still is unclear and most likely depending on the state of the cell cycle. Equally important is the elucidation of potential cytotoxicity and cellular damage caused by variations in size, purity, concentration and functionalization of CNTs. | |
| "Every cell relies on the uptake (endocytosis) of materials like proteins, cytokines and even synthetic carbon nanomaterials, to perform its required cellular fate functions," Sabrina Jedlicka, Associate Professor of Bioengineering and Materials Science & Engineering and Associate Dean of Rossin College of Engineering at Lehigh University, explains to Nanowerk. | |
| "Studying this process in detail is an extremely challenging and thus extremely interesting goal in biophysics," adds Slava V. Rotkin, Frontier Professor of Engineering Science and Mechanics with an appointment in the Materials Research Institute at Penn State. "Therefore, endocytosis is of interest for bringing therapeutic targets into cells. Studying the pathways of how materials get into the cell can aid in untangling trafficking to design higher efficiency targeted drug and gene delivery therapies." | |
| Identifying novel ways to differentiate and maintain healthy cell populations could lead to new therapies in regenerative medicine. Nanomaterials could be the key to this, but researchers still know too little about the detailed mechanisms underlying nanomaterial-cell interactions to safely design treatment protocols. | |
| In a further step towards unraveling how exactly synthetic nanomaterials interact with cells, Jedlicka at Lehigh University, Rotkin at Penn State, and Prof. Tetyana Ignatova at University North Carolina at Greensboro and their teams, conducted a series of experiments that allowed them to determine the mechanisms of cellular intake of DNA-wrapped single-walled carbon nanotubes (SWCNTs), and how they depend on the cell cycle (i.e. the state of the cell as it divides). | |
| They published their findings in Biophysical Reports ("Cell cycle-dependent endocytosis of DNA wrapped single-walled carbon nanotubes(DNA-SWCNT) by neural progenitor cells"). | |
 |
|
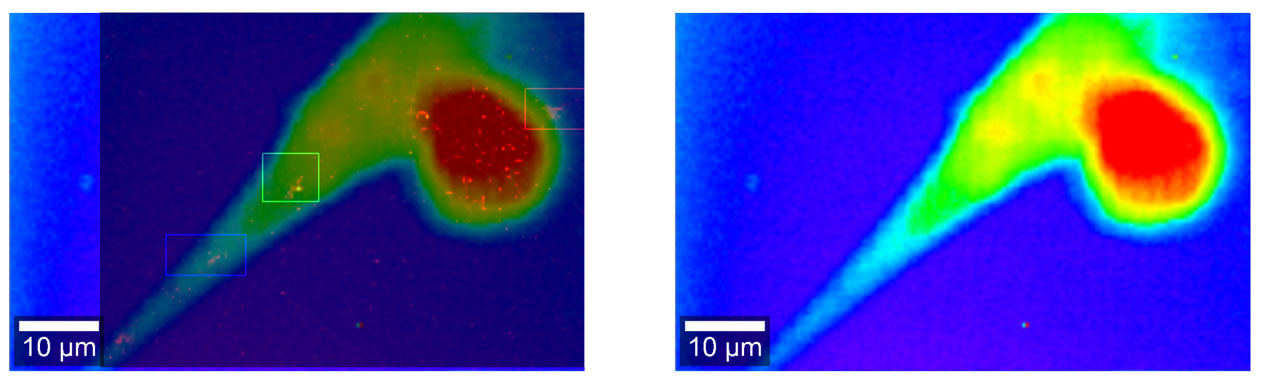
| Raman microscopy image of a fixed neural stem cell (right) overlaid with the Raman image showing three SWCNTs (left, highlighted with boxes around each SWCNT location). (Image courtesy of the researchers) | |
| The material used in this study has already been thoroughly characterized: CoMoCat-synthesized SWCNTs wrapped with (GT)20 single-stranded DNA oligomers. The DNA-conjugation serves as a biomolecule mask to the cells – even though this artificial strand would not be recognized by the cell – also substantially increasing nanomaterial solubility in aqueous buffer and inhibiting nanotube coalescence. | |
| "We hypothesized that the presence of the nanotubes, in optimized concentrations and external conditions, can up-regulate aspects of natural cellular fate processes that can be harnessed to further understand the system, which can be used in the future to develop improved nanosensors and delivery therapeutics," Jedlicka and Rotkin explain the background to this work. "This requires a cellular and molecular scale analysis of the nanotube-cell system in order to comprehend the modification of cell developmental behavior in response to potential nanomaterial-based therapies as well as the downstream implications of cell-material interactions on these therapies." | |
| The team's study focused primarily on the modes of entry of SWCNTs into cells and how internalization changes based on the phase state of the cell. Notwithstanding all the research that has been done in this field, detail aspects of endocytic mechanisms and pathways are still not fully understood. To the extent that scientists know about SWCNT-cell interactions, this information has mostly been either averaged over entire cell populations where the cells have a different age (i.e. at what stage each cell is in the cell cycle) or taken on a single cell with random age. | |
| "These entry mechanisms are accompanied by respective stimuli that trigger various downstream cellular responses and signal transduction changes in the cell accordingly," Jedlicka points out. "Unsurprisingly, this results in biochemical changes to the cell, vital to its growth and development, in addition to the cell's innate dynamic internal component reorganization." | |
| The researchers' overall results in this study strongly correlate with the hypothesis that nanomaterials gain entry into the cell by more than just one method, and that more than one method of endocytosis can be responsible for the uptake of a single cargo. | |
| Going forward, the team's next study will be focused on the mechanisms by which CNTs influence cell differentiation. This study will examine traditional means of cell differentiation in vitro compared to CNT-driven differentiation. This will allow an assessment as to whether the mechanisms by which nanotubes interact with cells are similar to the natural internal drivers of cell differentiation, thus allowing for a mapping of the signaling pathways and assessment of any potential challenges around gene up/down regulation. | |
| "Extension of our approach to other types of cells and other types of nanomaterials is certainly the right way of moving towards these goals," Rotkin concludes. "This is a very large volume of work, which our groups can't do on our own. It is up to the scientific community to contribute to this field." | |
| "As for ourselves" he adds, "we would like to get a deeper knowledge of the processes inside the cell, potentially using high-resolution, non-destructive optical characterizations techniques such as Raman microscopy (see for instance: "Micro-Raman spectroscopy as an enabling tool for long-term intracellular studies of nanomaterials at nanomolar concentration levels ") and Scattering Scanning Near-field Optical Microscopy (sSNOM) (see for instance: "Multidimensional Imaging Reveals Mechanisms Controlling Multimodal Label-Free Biosensing in Vertical 2DM-Heterostructures")." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
