| Jul 07, 2022 | |
Investigating water/oil interfaces with opto-thermophoretic tweezers |
|
| (Nanowerk Spotlight) Spontaneous charging of interfaces between water and hydrophobic (i.e., water-repelling) media is a mysterious feature whose nature and origin – despite many efforts to explain them – are still not fully understood. For instance, experiments reveal that water interfaces with immiscible liquids such as oil droplets, solid hydrophobic polymers, hydrophobic assembled structures, and even gas bubbles are usually negatively charged (they become positively charged in highly acidic solutions). | |
| "Many spectroscopic results and interpretations along with computational work are not consistent with one another and a consensus on the nature and origin of interfacial charging has not been reached," Youngsun Kim, a graduate student in the Zheng Research Group at The University of Texas at Austin, tells Nanowerk. "We believe that a clear understanding of this puzzling phenomenon needs a variety of approaches from diverse perspectives, which, hopefully will all add up and be pieced together towards a complete description of the full picture." | |
| Kim is first author of a paper in Nature Communications ("Investigating water/oil interfaces with opto-thermophoresis") that represents one such approach. In this work, the team used the opto-thermophoresis of oil droplets to obtain the dynamic characteristics of interfaces. | |
| "The use of opto-thermophoretic tweezers to analyze surfactant-free perfluoropentane (PFP)-in-water emulsions as a model revealed that water molecules and mobile negative charges are present at the water/oil interfaces with specific dipole arrangement to hydrate oil droplets, and that this arrangement is highly susceptible to thermal perturbation due to the mobility of the negative charges," Kim explains. | |
 |
|
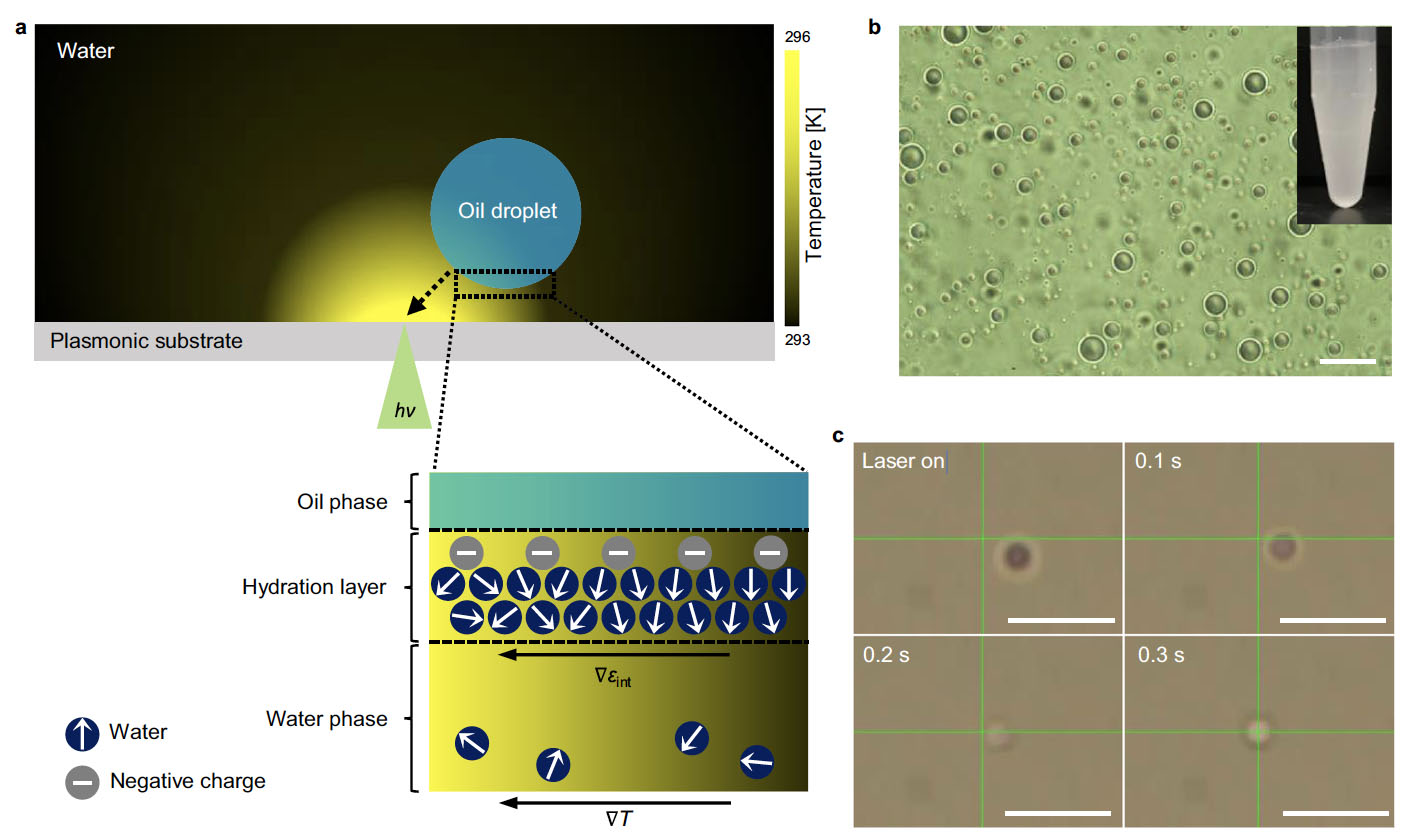
| Working principle of opto-thermophoretic tweezers and trapping of a PFP droplet. a A temperature gradient is created by the photothermal conversion of light at a plasmonic substrate, and a water-dispersed oil droplet migrates to a hot region. Water molecules and negative charges are arranged at the water/oil interface, having a radial orientation of water dipoles (indicated by arrows). The temperature gradient (⊽T = dT/dr, r: distance from the laser beam) induces a permittivity gradient (⊽εint) along the interfacial water layer by thermal perturbation of interfacial water ordering, leading to a thermophoretic migration of the droplet toward the hot region. b Optical image of PFP droplets dispersed in water (scale bar: 10 µm). Inset is a photograph of PFP-in-water emulsions. c Optical images of a PFP droplet under an optical input in the course of time (scale bars: 10 µm). Crossbars indicate the position of the laser beam. The droplet is trapped once it is close to the laser beam. (Reprinted from Nature Communications under a Creative Commons 4.0 International License) | |
| The researchers' findings are based on the capability of opto-thermophoretic tweezers – which operate under a microscale thermal field created by a laser beam irradiated on a photothermal substrate – to create a microscale temperature gradient and to reflect information of interfacial dipole arrangement. By measuring the opto-thermophoretic properties of oil droplets and corresponding theoretical analysis, the team derived the interfacial behavior of water and charge. | |
| Previous studies including electrokinetic, spectroscopic, and computational methods relied on the static analysis of aqueous interfaces. The present work advances the understanding of the interfacial charging by providing a complementary perspective in terms of the thermal responsiveness of interfacial dipole arrangement of water and charge. | |
| This work also suggests the use of potential of opto-thermophoretic tweezers for further elucidation of the various roles of water at interfaces present in many biological and chemical systems – cell membrane dynamics and function, protein folding, and electrochemical reactions. | |
 |
|
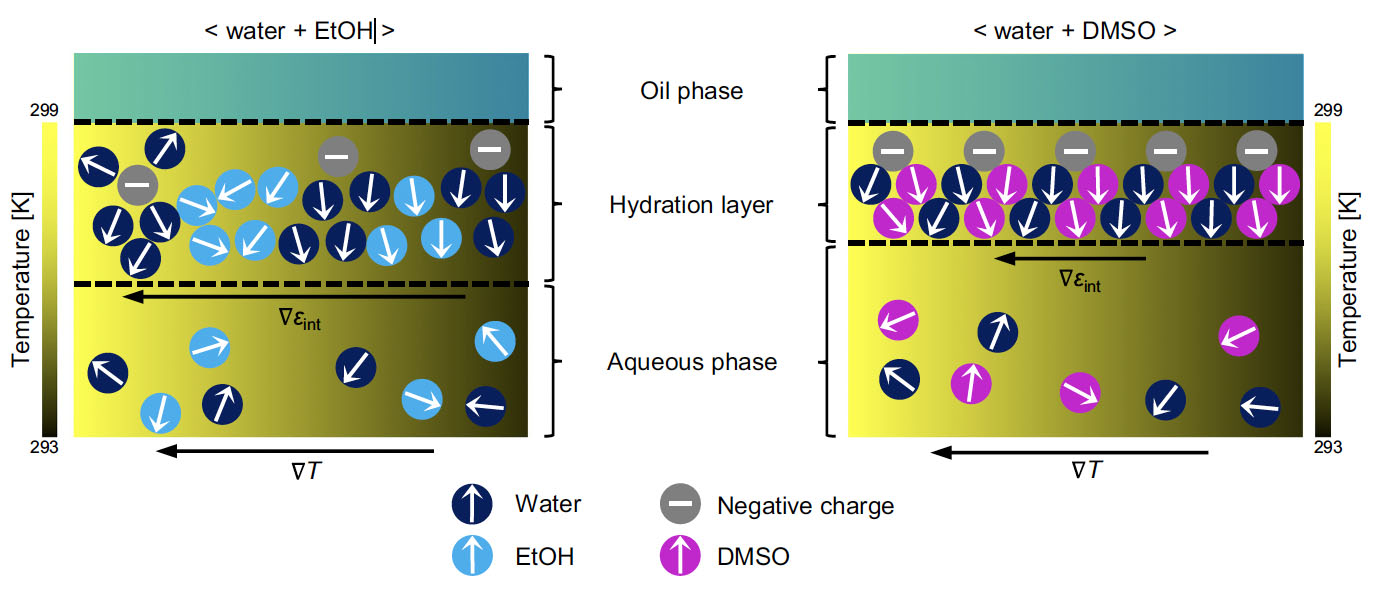
| Schematic illustration of the hydration layer in different solvent environments. Ethanol (EtOH) destabilizes negative charges owing to weak dipole-charge interactions and weak hydrogen bonding with water, forming a diffuse, inhomogeneous, and therefore, thermally responsive interface. The strong complexation of dimethyl sulfoxide (DMSO) with water constructs a rigid interface that reduces its thermal responsiveness. (Reprinted from Nature Communications under a Creative Commons 4.0 International License) | |
| The researchers plan to extend their opto-thermophoretic analyses to other aqueous interfaces such as those with air, hydrocarbon oils, and relatively polar oils to further study contributions of other factors to interfacial structures. | |
| "Even now, the identity of ionic species, responsible for interfacial charge, and their specific complexation with water is still in question, thus calling for further studies in which opto-thermophoresis is combined with spectroscopic and computational techniques to elucidate phenomena pertinent to the interfacial behavior of water," Kim concludes. "In addition, the capability of manipulating, assembling, and merging droplets in liquids will allow a wider range of applications of opto-thermophoretic tweezers, including controllable microreactors and reconfigurable microfluidics." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
