| Posted: Jun 25, 2008 | |
Self-assembly gets more sophisticated with control over anisotropic shapes |
|
| (Nanowerk Spotlight) Nanotechnology's much-touted notion of bottom-up fabrication – the key concept behind every idea and model of advanced nanotechnologies – so far doesn't have much to do with assembly-line style of manufacturing; rather, it relies on natural self-assembly processes. The stability of covalent bonds enables the chemical synthesis of almost arbitrary configurations of up to 1000 atoms. Larger molecules, molecular aggregates, and forms of organized matter more extensive than molecules cannot be synthesized bond-by-bond. Self-assembly is one strategy for organizing matter on these larger scales (Source). | |
| Nevertheless, as a wholly novel way to manufacture and create new materials, self-assembly is of fundamental importance for the future of a myriad of technologies. While there is no doubt self-assembly works, as evidenced by the world around us, scientists have just begun to understand and devise working examples of self-assembly. Much of this work has been at the millimeter and micron scales were it is relatively easy to fabricate components for assembly. A recent paper details a general method, using microcontact printing, for modifying cubic building blocks with nanoscale dimensions. The controlled assembly of metallic nanoparticles remains a challenge and this work provides a novel functional example to study and build upon. | |
| "While other studies have reported assembly of two- and three-dimensional structures from cubic components, the procedure we outline in our recent paper is more general and relies on the ubiquitous and adaptable hydrophobic force," Dr. Younan Xia tells Nanowerk. "Our study suggests that the hydrophobic force can be controlled to make novel self-assembled nanostructures through anisotropic interactions in solution and at the air-water interface and, furthermore, it verifies the versatile and practical nature of anisotropic self-assembly systems." | |
| Xia, the James M. McKelvey Professor in the Department of Biomedical Engineering at Washington University in St. Louis, together with his team has published his findings in a recent paper in Advanced Materials ("Controlling the Assembly of Silver Nanocubes through Selective Functionalization of Their Faces"). | |
| "While the use of hydrophobic and hydrophilic self-assembled monolayers (SAM) in self-assembly processes are known, our example is notable in the way we programmed the components and used both hydrophobic and capillary interactions for assembly," explains Xia. "This gave us the control to assemble structures with different geometries and sizes. Furthermore, the scale and the extent of the assembly were unusual as we could assemble 100 nm cubes into a three-dimensional cubic array over micron distances, which is something not demonstrated previously." | |
| He points out that the key concept of self-assembly is that the final structure is usually predetermined by the characteristics of the building blocks: the information that defines a self-assembly process – and thus the final structure – is often coded in the building blocks in the form of shape, topology, or surface functionality. | |
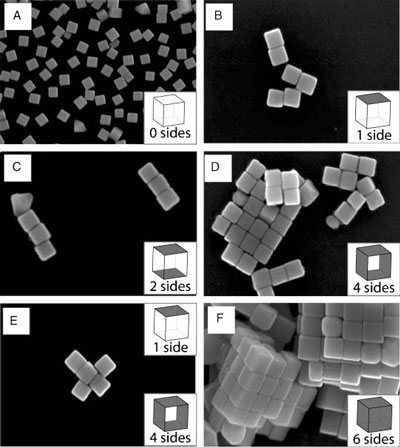
| From the possible combinations of hydrophobic and hydrophilic faces, Xia's team generated five distinct SAM-modified silver nanocubes and assembled them into four different nanostructures and one microstructure. | |
 |
|
| SEM images of silver nanocubes and the assemblies. Unfunctionalized cubes deposited on silicon from water are shown in (A) for reference. Nanocubes whose faces have been selectively functionalized with hydrophilic and hydrophobic thiolate SAMs and then allowed to assemble in water are shown in (B–F). The number of faces on each cube that were rendered hydrophobic is indicated in the bottom right corner of each panel, the remaining faces on the cube were rendered hydrophilic. In (E), cubes with four hydrophobic sides were mixed with cubes that only had one hydrophobic face at a ratio of 1:4 and then allowed to self-assemble in water. All cubes used in this study had a mean edge length of (97±6) nm (as determined from 123 cubes). (Reprinted with permission from Wiley-VCH Verlag) | |
| "The assembled structures are expected if the silver nanocubes come into contact with their hydrophobic faces adjacent and parallel" says Xia. "These building blocks come together due to a hydrophobic driving force that is proportional to the solvation free energy of the water surrounding the SAM-functionalized silver nanocube. The solvation free energy is primarily enthalpic and dependent on the number of hydrophobic and hydrophilic faces on each cube. The silver nanocubes spontaneously assemble to reduce their solvation free energy by reducing their hydrophobic surface area exposed to water." | |
| The procedure appears to be limited only by the number of silver nanocubes available and the size of the silicon substrate, and could practically be scaled up to generate large quantities of self-assembled structures for use in surface-enhanced Raman scattering (SERS). | |
| "Aggregates of metal nanoparticles can lead to the detection of single molecules when coupled with SERS, but such detection has been troubled by reproducibility issues as the aggregates are poorly defined and hardly controllable" says Xia. "SERS requires control over nanoscale features and geometry and the well-defined structures described in our work hold great promise for addressing this technical problem." | |
| He also notes that this approach can be extended to form dimers, trimers and other assemblies where hot spots (areas of high Raman signal enhancement) reside between nanoparticles. Such structures could contribute to the understanding of SERS and provide a system that is capable of detecting only a handful of relevant molecules. | |
| The motivation behind this work underscores an important factor for the future of nanoscale self-assembly systems: new components will give rise to unique assembled structures and original procedures for programming assembly. | |
| While Xia's work doesn't solve a specific problem, it confirms that some of the concepts (e.g., self-assembly via hydrophobic or capillary interaction) that have been demonstrated with microscale or molecular scale building blocks also work for nanoscale building blocks. Basically, this work fills a gap between the molecular and micron scales. | |
| In order to develop sophisticated self-assembly techniques for nanoscale components, one of the challenges lies in breaking the isotropy of the system in terms of its components and interactions. The usefulness of these systems will ultimately be determined by the complexity of the components and currently most examples of assembly deal with spherical particles acting isotropically. | |
| Over the past several years, a diverse collection of nanoscale building blocks with anisotropic shapes has also emerged, as well as schemes to add directionality to their interactions through selective functionalization. | |
| "This has been a necessary and very rewarding first step, however, the future of nanoscale assembly lies with non-spherical, anisotropic components" says Xia. "It remains a grand challenge to design and fabricate nanoscale building blocks that can interact anisotropically to form self-assembled structures deviated from close packing. Fabricating these components and programming their interactions through surface modification or some other route will provide the technology needed for the next generation of sensors, electronics, and other devices and materials." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
