| Jul 13, 2023 | |
Rapid at-home molecular diagnostics is the future of preventive medicine |
|
| (Nanowerk Spotlight) Advanced molecular diagnostics tests that can rapidly and precisely detect diseases within the home could revolutionize preventive medicine and improve global biosecurity. Nucleic acid amplification tests (NAATs) like polymerase chain reaction (PCR) provide high sensitivity and specificity for infectious disease identification by amplifying pathogen DNA and RNA. | |
| However, centralized lab-based NAATs require complex, tedious sample processing steps like pathogen lysis, nucleic acid purification, and enzymatic amplification. These manual procedures rely on trained personnel operating expensive equipment, limiting accessibility. | |
 |
|
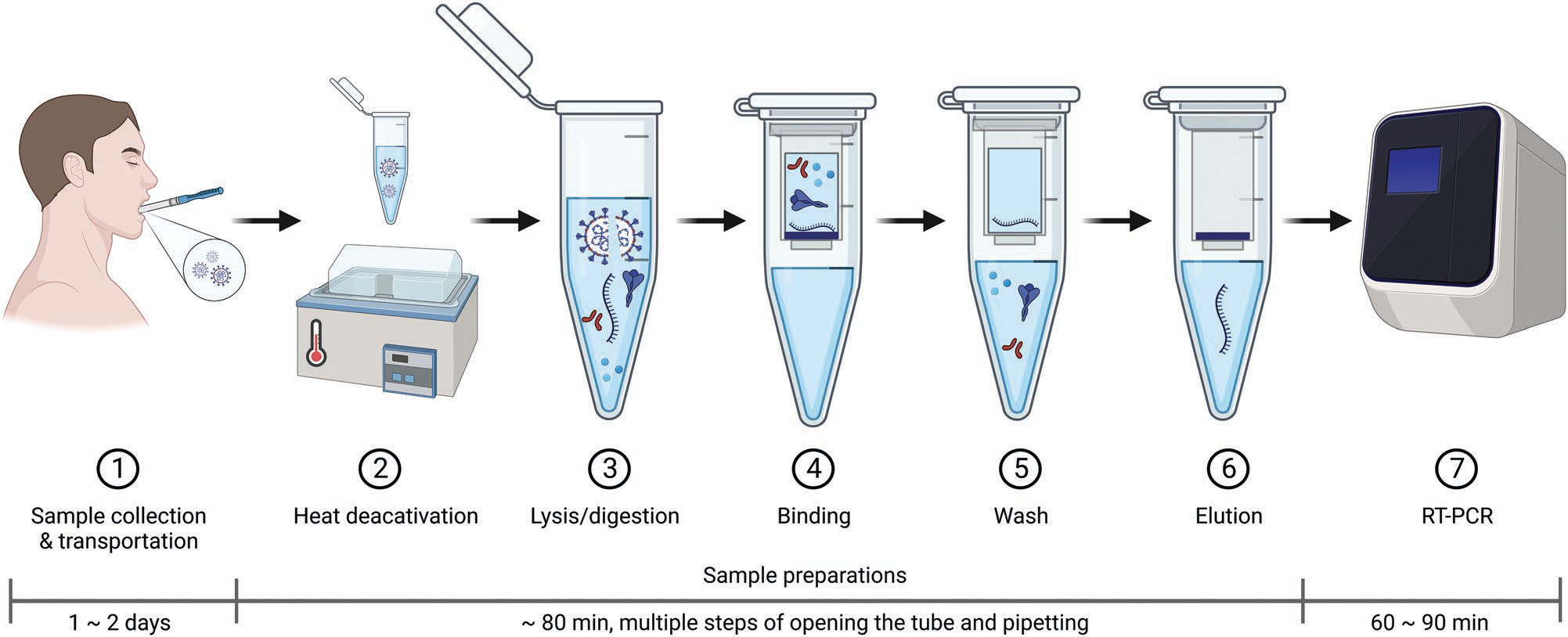
| Conventional laboratory protocol of spin-column-based nucleic acid extraction for molecular diagnostics for COVID-19. Step 1: sample collection and transportation. Biological specimen types are collected by various collection procedures, such as a nasopharyngeal swab, nasal swab, and oropharyngeal swab, and transported to the centralized laboratories as soon as possible after collection. Of particular interest for molecular epidemiology analysis are those by which the samples can be collected most conveniently and effectively at the lowest cost. Step 2: heat deactivation. The viruses are deactivated by incubating the clinical sample at an elevated temperature to destabilize viral proteins and assemblies, rendering them incapable of infection during the downstream manual operation. Step 3: lysis/digestion. Lysis buffer contains a high concentration of chaotropic salts and detergents. Chaotropes disrupt hydrogen interactions and lead to the destabilization of proteins and nucleases. Organic amphipathic detergents break up the cell membrane structure by separating membrane proteins with the hydrophobic part of detergents from membranes. Proteases can be included in the lysis buffer to digest the contaminating proteins and degrade the nucleases. The lysis buffer shows higher efficiency at elevated temperatures. Step 4: binding. The lysate is transported to a spin column. The chaotropic salts provide favorable conditions for nucleic acid transfer to the silica membrane of the spin column by creating a hydrophobic environment to break down the association between NAs and water. Meanwhile, chaotropes provide positively charged cations to saturate the silica membrane, thus improving the absorption of negatively charged phosphate backbones of NAs under hydrophobic conditions. Since NAs are insoluble in ethanol, the addition of ethanol will enhance nucleic acid precipitation to the silica membrane. Step 5: wash the washing buffer, with ethanol as the dominant component, removes impurities such as protein polysaccharides residues. Multiple washing steps are usually conducted to thoroughly remove residual contaminations and buffer solutes. Residual ethanol should be avoided after the washing step since it may prevent the sequent elution of NAs and inhibit nucleic acid amplification. Step 6: elution. The elution buffer or pure water at pH 8–9 is typically used to release the NAs from the silica membrane to the bottom of the centrifuge tube during centrifugation. Step 7: reverse transcription-polymerase chain reaction (RT-PCR). The purified RNAs are reverse-transcribed to complementary DNA (cDNA), followed by cDNA amplification and fluorescence detection. (Reprinted with permission by Wiley-VCH Verlag) | |
| Integrated microfluidic platforms that combine automated sample preparation with on-chip NAATs in a single miniaturized device offer a practical solution to perform precise molecular diagnostics at home without a dedicated lab. | |
| A review in Advanced Materials ("Toward Rapid and Accurate Molecular Diagnostics at Home") evaluates recent advances in integrated sample preparation for NAATs using common clinical samples like blood, urine, saliva, and stool. | |
| Blood provides an abundance of genetic and infectious disease biomarkers. However, invasive sampling via venipuncture or fingerprick is required to acquire blood. An integrated microcapillary loop-mediated isothermal amplification (LAMP) method successfully extracted DNA from a tiny 200 nL whole blood sample obtained by minimally invasive fingerprick, avoiding the need for costly and painful traditional blood draws. | |
| Urine offers a completely noninvasive liquid biopsy, in contrast to blood. However, urine contains far lower abundances of nucleic acid biomarkers. Therefore, microfluidic magnetic bead platforms are necessary to capture and concentrate scarce pathogen DNA/RNA from large urine volumes prior to extraction. Concentration improves detection limits. | |
| Saliva and nasal swabs enable noninvasive sampling of respiratory infections through virus particles shed in mucus. An integrated microfluidic cartridge extracted HIV ribonucleic acid (RNA) biomarkers from only 30 μL of saliva in just 10 minutes, demonstrating the potential for extremely rapid and sensitive sample-to-answer NAATs. | |
| Stool contains gastrointestinal pathogens and can noninvasively identify lower digestive tract infections. However, viscous stool samples require mechanical homogenization on-chip prior to pathogen capture and nucleic acid extraction. | |
| Recently, an ingenious sharp-edge acoustofluidic system actively homogenized stool inside microchannels by generating strong streaming forces with ultrasonically oscillating microscopic structures micromachined out of silicon. | |
| Repurposable integrated microfluidic chips flexibly process diverse clinical sample types on a common platform: | |
| Microfluidic channels packed with silica microbeads extracted purified RNA from raw cellular lysate prior to on-chip nucleic acid sequence-based amplification (NASBA) detection without cross-contamination between samples. | |
| Self-powered integrated blood analysis chips successfully integrated plasma separation from tiny 5-10 µL whole blood specimens with downstream multiplex immunoassays detecting protein biomarkers. | |
| Modular microfluidic platforms enabled interchangeable single-use assay cartridges tailored for specific sample types like sputum or blood, providing versatile tools for a wide range of nucleic acid and protein diagnostic testing. | |
 |
|
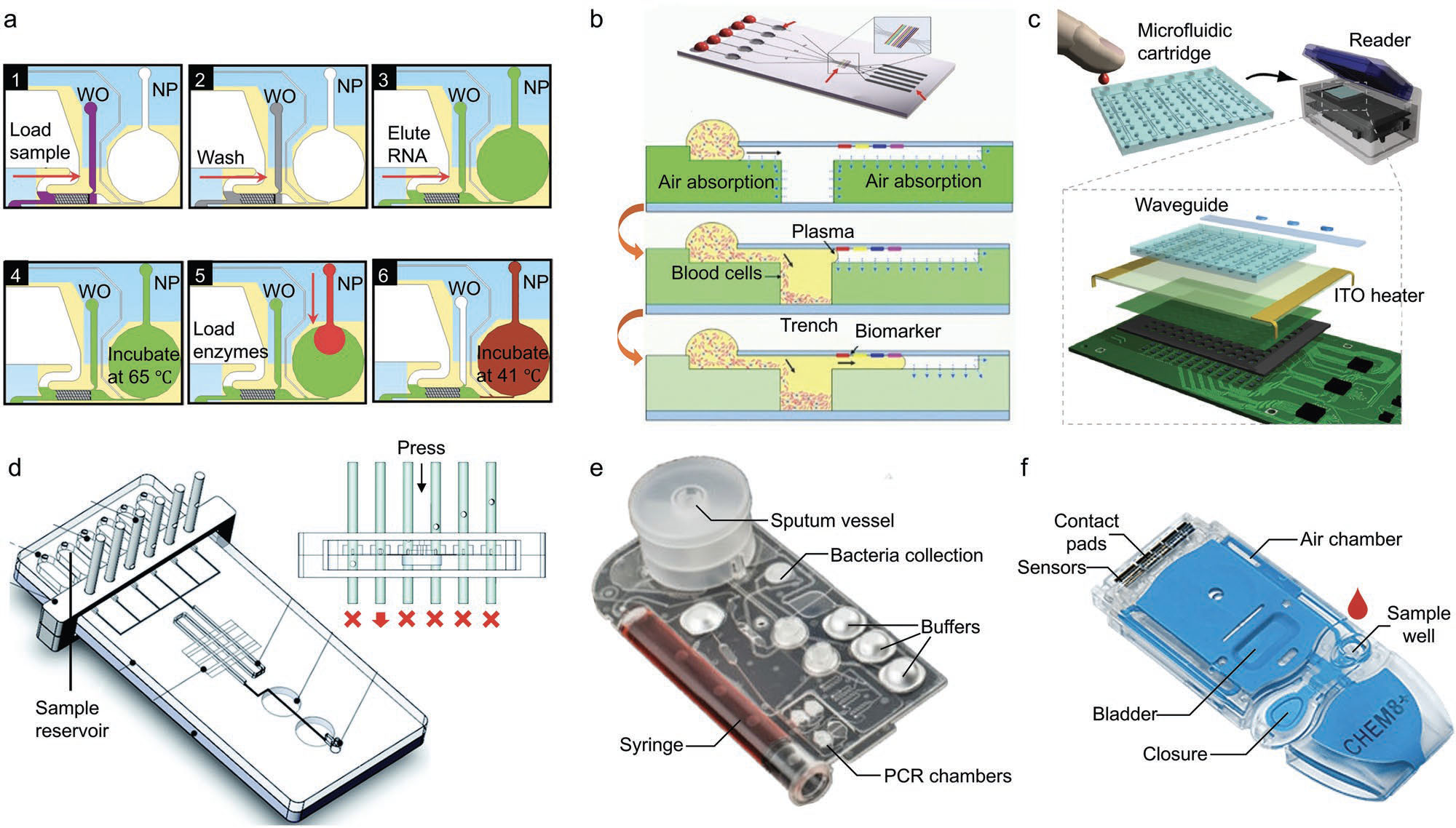
| Repurposable integrated on-chip sample preparation with various biological samples. a) Integrated microfluidic RNA purification chamber and real-time NASBA device. 1: Sample load; 2: buffer wash; 3: RNA elution; 4: denaturation of secondary and tertiary RNA structures; 5: NASBA enzyme load; 6: amplification process. b) Stand-alone self-powered integrated microfluidic blood analysis system. The blood is driven into the microchannel by degas-driven flow, and the plasma is separated by trapping red and white blood cells in an integral trench structure. c) Microfluidic biomolecular amplification reader (µBAR), including the microfluidic cartridge and the monitor. d) On-chip valve-assisted microfluidic chip demonstrating the automation of reagent control. A mechanical slider controls the downward movement of the on-chip valves to open and close the channels, and the reagents flow through the chamber of interest in sequence. e) A universal microfluidic cartridge for M. tuberculosis (MTB) diagnostics from sputum sample. f) i-STAT microfluidic cartridge comprising thin-film electrode sensors on silicon chips to detect targets. The air bladder is depressed by the motor in. (Reprinted with permission by Wiley-VCH Verlag) (click on image to enlarge) | |
| On-chip NAATs offer numerous advantages over conventional benchtop laboratory testing methods: | |
| Portability, automation, and speed – Thin-film microfabricated heaters integrated inside microfluidic chips enabled rapid thermal cycling to support on-chip PCR DNA amplification. | |
| Bubble prevention – Ingenious bubble-free microfluidic PCR chips prevented vapor bubble formation using impermeable polyethylene vapor barriers and integrated on-chip vacuum channels for bubble removal. Bubbles disrupt nanoliter reaction volumes. | |
| Photonic heating – Patterned gold films deposited on chips enabled precise photonic heating by efficiently converting light into heat to achieve rapid thermal cycling without contact to cumbersome heating blocks. | |
| Digital microfluidic partitioning – Microfluidic digital PCR enhanced analytical sensitivity by partitioning minute samples into thousands of 50-250 nL wells to detect single DNA molecules, greatly simplifying integration with upstream sample preparation compared to complex laboratory quantitative PCR instruments. | |
| At-home NAATs paired with automated integrated microfluidic sample preparation can enable frequent population-wide screening during viral outbreaks, providing invaluable epidemiological data to halt diseases in their tracks. Their convenience and accessibility in low-resource settings is absolutely crucial for improving preventive medicine on a global scale. In the not-too-distant future, flexible wearable microfluidic biosensors may provide on-the-spot molecular diagnostic capabilities using microliter volumes of easily accessed body fluids like sweat or saliva. | |
| In summary, innovative lab-free microfluidic NAAT platforms are bringing the powerful capabilities of advanced nucleic acid testing directly into the average consumer’s home, workplace, or even pocket to provide on-demand, decentralized diagnostics that can help stop outbreaks before they have a chance to spread. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
