| Aug 01, 2023 | |
'Drycells' promise to transform single cell research |
|
| (Nanowerk Spotlight) Cell culturing demands the ability to isolate and analyze individual cells, a requirement that is especially critical in tissue engineering, regenerative medicine, and gene therapy. Cell culturing involves growing cells in controlled conditions outside their natural environment, and is a fundamental technique used in biological research and medical applications. | |
| However, studying cells at the individual rather than aggregate level presents challenges. Researchers need tools to reliably pick, separate, and manipulate single cells from cultures. This enables examining cell heterogeneity, identifying rare cells, and tracking dynamic single-cell behaviors. | |
| New innovations in single-cell isolation and handling are essential to overcome barriers in tissue engineering, stem cell therapies, and precision medicine. But existing cell-picking approaches like flow cytometry and laser capture microdissection have limitations in throughput, cost, or ease-of-use. There is a need for accessible, efficient means to isolate individual cells in a minimally invasive way. | |
| A recent paper in Advanced Materials ("Drycells: Cell-Suspension Micro Liquid Marbles for Single-Cell Picking") from researchers at the National Institute for Materials Science in Japan introduces “drycells”, a versatile new tool to enable flexible single-cell picking and analysis. | |
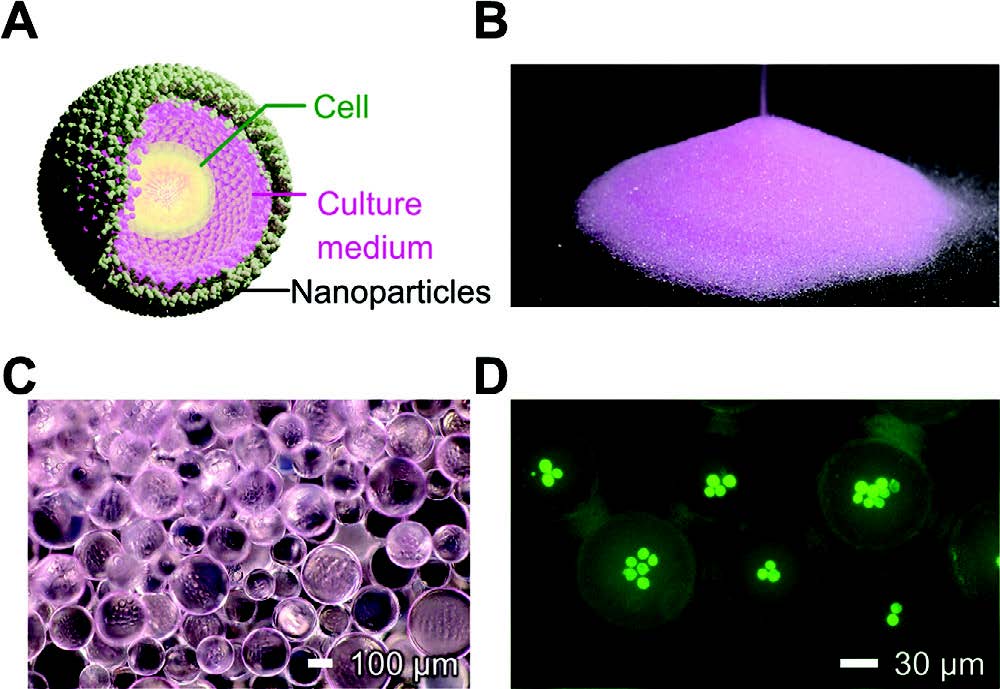 |
|
| A) Drycell structure. B) Mass-produced drycells behave like powder. C) Optical microscopy image. D) Fluorescence image of drycells. The encapsulated F-tractin-EGFP-expressing NIH-3T3 cells fluoresced in green. (Reprinted with permission by Wiley-VCH Verlag) | |
| Drycells are tiny droplets encapsulating single cells, created via a simple yet powerful production process. Their unique properties allow precise control over single-cell encapsulation and manipulation. This approach could help drive advances in diverse areas relying on cell culturing and single-cell techniques. | |
| Drycells offer a simple, minimally invasive way to encapsulate individual cells in stable microdroplets. To create drycells, the researchers spray a cell suspension onto a bed of hydrophobic silica nanoparticles. The nanoparticles coat the surface of the droplets, preventing them from sticking together. This creates microdroplets 10-300 µm in diameter, each encapsulating an aqueous culture medium with one or more cells inside. | |
| Remarkably, even with over 95% water content, the drycells behave like a dry powder – hence the name. They can be sieved to control droplet size and number of encapsulated cells. The researchers showed they could produce a high yield of droplets with just a single cell inside. They also encapsulated defined pairs like one normal and one cancer cell. The number of cell colonies per droplet can be tuned as well. | |
| A key advantage of drycells is their mechanical stability despite the high internal pressure from their small size. The nanoparticle shell resists droplet coalescence better than a continuous film because nanoparticles can shift around fluidly. Drycells withstand sufficient compression and centrifugal forces for practical handling, unlike standard liquid marbles. | |
| Drycells exhibit impressive flexibility and reconfigurability too. Their shells self-heal after being pierced and can undergo splitting, merging, or liquid exchange without rupturing. This enables manipulating and modifying encapsulated cells using tools like micropipettes. Cells can even be extracted from drycells after storage and return to normal growth. | |
| When tested with fibroblasts, the cells remained rounded up rather than adhering and spreading inside the drycells. The researchers suggest nanoparticle mobility at the droplet interface fluidizes the surface, preventing stable cell adhesion. This could allow easy storage and transfer of adhesive cells. | |
| Initial results show high cell viability after drycell encapsulation and storage. After 3 days, over 80% of the cells extracted from drycells remained alive and healthy. This demonstrates the gentle nature of the drycell production process. | |
| In summary, the proposed drycells offer a versatile new tool for single-cell research. Their simple production, mechanical stability, handling flexibility, and biocompatibility enable reliable single-cell isolation and analysis. By tuning droplet size and cell density, researchers can encapsulate defined numbers or types of cells with ease. | |
| Drycells have potential uses ranging from tissue engineering to cancer research. This accessible approach could help advance single-cell biology and overcome barriers to entering the field. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
