| Aug 29, 2023 | |
New fabrication method creates biomaterials with layered architecture to advance tissue engineering |
|
| (Nanowerk Spotlight) Tissue engineering aims to restore damaged tissues by combining cells, engineering materials, and biologically active molecules. The architecture and composition of the engineered scaffolds that support cell growth are critical for mimicking native tissues. Advances in 3D printing and assembly of multiple materials are enabling more life-like synthetic scaffolds. | |
| Researchers have been grappling with how to create materials that can not only mimic the complex structures found in biological tissues but also promote cellular growth and function. This fabrication of biomaterials with nanoscale features and a layered composition remains challenging. | |
| A recent study, published in Advanced Functional Materials ("Fabrication of Architectured Biomaterials by Multilayer Co-Extrusion and Additive Manufacturing"), has taken a significant step forward in this direction by employing a top-down co-extrusion approach to generate thin alternating polymer layers with bottom-up 3D printing to form intricate structures. | |
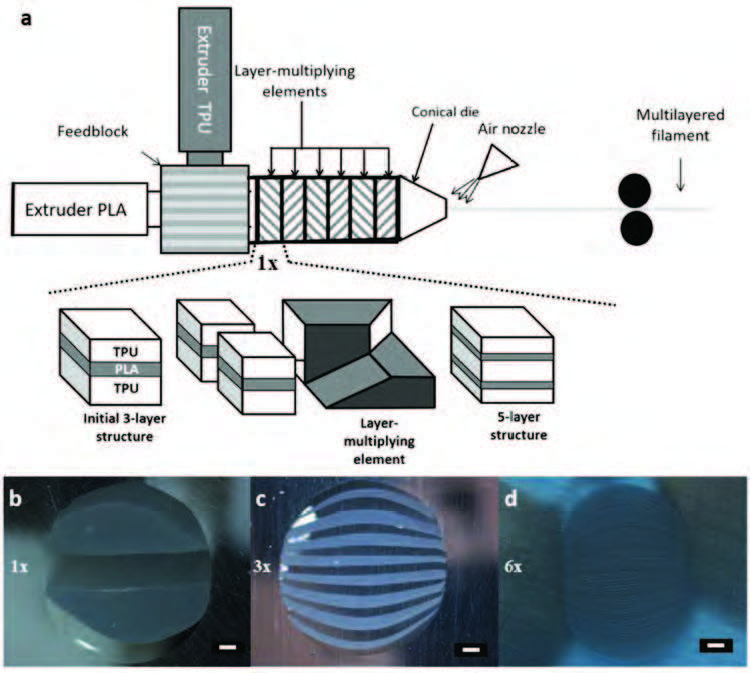
| In this new work, researchers from Monash University, Australia and Arts et Metiers Institute of Technology, France developed a two-step fabrication process. First, they used a multilayer co-extrusion technique to generate thin alternating layers of biodegradable poly(lactic acid) (PLA) and elastic thermoplastic polyurethane (TPU) with precisely controlled thickness and composition. The viscous polymer melts were forced together under shear to create a layered filament with up to 129 alternating PLA and TPU layers. | |
 |
|
| The fabrication of multilayer filaments using co-extrusion and linear multiplying elements. a) Schematic diagram showing the multilayer co-extrusion process by forced assembly of two polymer melts through a series of layer multiplying elements (LMEs). The expanded section shows the process for 1 multiplier (1×), which is repeated to achieve a higher number of layers; and b–d) Cross sectional surface optical micrographs of multilayered co-extruded PLA/TPU filaments produced with b) 1, c) 3, and d) 6 LMEs. Scale bar, 200 µm (b–d). (Reprinted with permission by Wiley-VCH Verlag) | |
| The team used this multilayered filament as the feedstock material for fused deposition modeling 3D printing to build tissue engineering scaffolds with complex shape. Extensive analysis showed that the layered architecture was preserved during printing, with continuous interfaces between the polymer phases. By tuning the co-extrusion process, they produced filaments with individual layer thickness ranging from around 14 to 350 microns. This enabled 3D printed constructs with nanoscale polymer layers. | |
| Mechanical testing revealed that certain architectures led to improved elongation and ductility compared to the pure polymer components. The combination of stiff, brittle PLA and flexible TPU in an optimized multilayered structure enhanced the overall performance. | |
| The researchers explored the potential of polymer filaments with alternating layers of PLA and TPU for cardiac tissue engineering. When rat heart muscle cells (cardiomyocytes) were grown on the 3D printed scaffolds, the multilayered structure strongly influenced the cell morphology, alignment and beating function. | |
| Cardiomyocytes are the muscle cells that make up the cardiac muscle tissue, and their proper growth and function are crucial for heart health. Traditional scaffolds often fall short in providing the necessary environment for these cells to thrive. However, the multilayered architecture of these new scaffolds showed promising results in improving both the morphology and functionality of the cardiomyocytes. | |
| In particular, scaffolds with an intermediate layer thickness of around 20 microns significantly improved cardiomyocyte maturation and synchronization over single polymer compositions. | |
| The study's approach is noteworthy for several reasons. First, the use of co-extrusion allows for the creation of filaments with multiple materials, each serving a specific purpose. PLA is known for its rigidity and biocompatibility, making it an excellent choice for structural support. On the other hand, TPU is more flexible and can mimic the elastic properties of biological tissues. By combining these materials, the researchers were able to create a scaffold that offers the best of both worlds: structural integrity and flexibility. | |
| Second, the application of 3D printing technology adds another layer of sophistication. 3D printing allows for precise control over the geometry of the scaffold, enabling the creation of complex structures that closely mimic natural tissues. This is particularly important for cardiac tissue engineering, where the arrangement of cells and fibers plays a crucial role in the tissue's mechanical properties and functionality. | |
| The study also opens doors for customization. Since both co-extrusion and 3D printing are highly controllable processes, it's possible to tailor the properties of the scaffold to meet specific requirements. This could be particularly beneficial in personalized medicine, where treatments are customized to individual patients. | |
| While the study focused on cardiomyocyte culture, the implications extend far beyond cardiac tissue engineering. The flexibility in material choice and scaffold design makes this approach applicable to a wide range of tissue engineering applications, from bone and cartilage regeneration to wound healing. Moreover, the study paves the way for further research into the optimization of scaffold properties, which could lead to even more effective biomaterials in the future. | |
| The significance of this work lies in its potential to revolutionize the way we approach tissue engineering. By integrating advanced fabrication techniques and material science, the researchers have created a versatile platform for developing next-generation biomaterials. These advancements could have a profound impact on healthcare, offering new avenues for the treatment of various diseases and conditions. While there is still much work to be done, this study serves as a compelling example of how interdisciplinary research can drive innovation in biomedical engineering. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
