| Oct 04, 2023 | |
Light-propelled bacteria power highly efficient biohybrid microbots (w/video) |
|
| (Nanowerk Spotlight) Microscopic robots smaller than the width of a human hair could one day travel through the human bloodstream to deliver targeted drugs or perform minimally invasive procedures. Creating robots at such small scales brings enormous engineering challenges, but researchers are increasingly looking to biology for solutions. | |
| In a new study published in Advanced Functional Materials ("Light Controlled Biohybrid Microbots"), scientists from Sapienza Universitá di Roma demonstrate biohybrid microbots that harness living bacteria as onboard engines that can be wirelessly steered using only light. | |
| Biohybrid microbots are an emerging class of microscopic robots that integrate biological components like cells or tissues with synthetic materials and structures. The field aims to harness the sensing, actuation, and energy generation capabilities of living cells to produce robotic devices on the micro and nanoscale. | |
| Conventional microbots built solely from inorganic materials face major challenges in miniaturization. Electronic controls, sensors, and actuators are difficult to fabricate and integrate at very small sizes. Biohybrid microbots seek to overcome these limitations by incorporating biological actuators like contractile heart or muscle cells and biological sensors such as neurons. | |
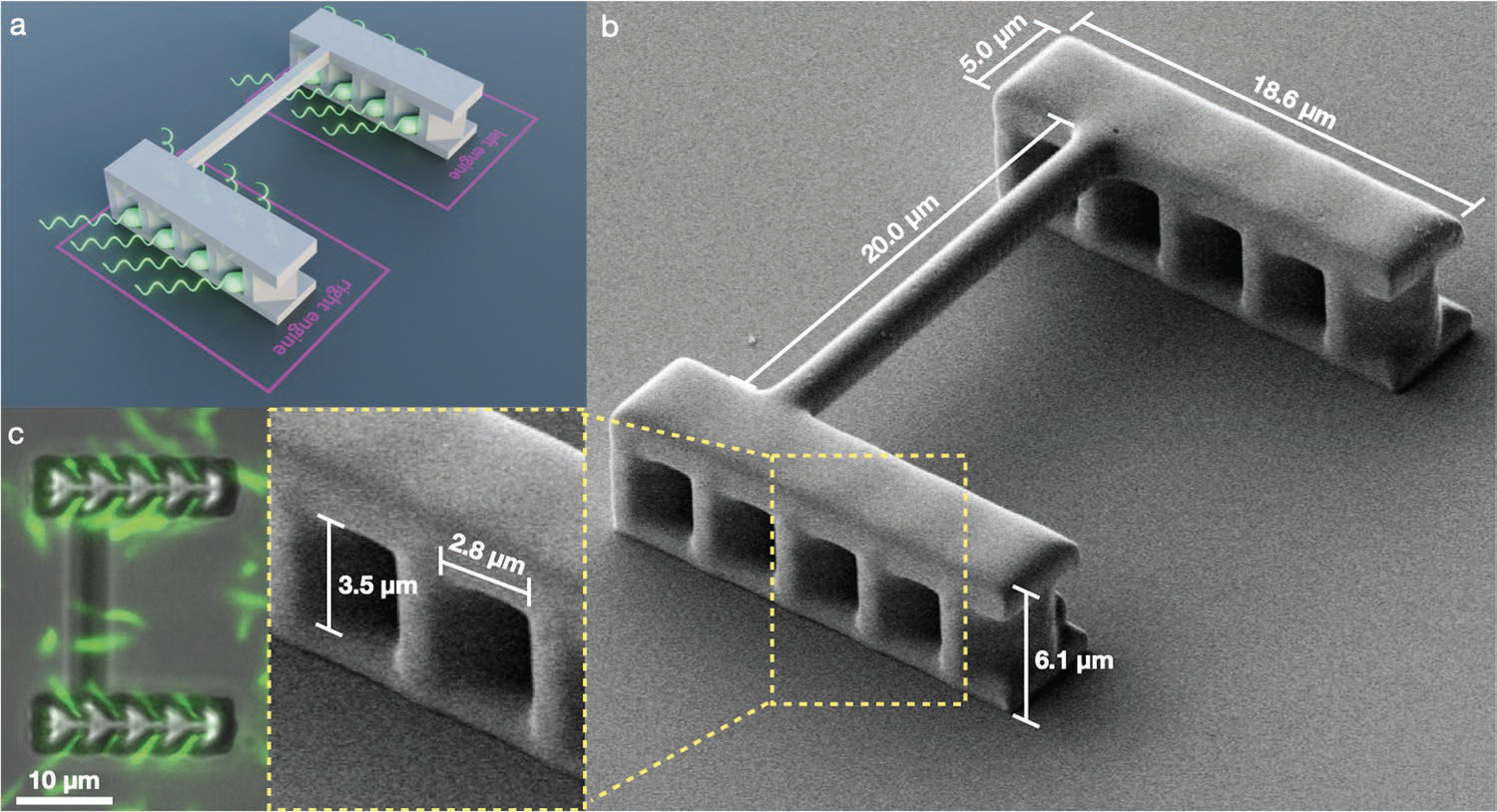
| In the new study, the researchers created biohybrid microbots using bacteria as tiny onboard engines. They designed a 3D-printed rigid plastic chassis for each microbot with chambers to trap individual motile E. coli bacteria in an aligned configuration. This interface transmits forces from the bacteria's rotating flagella out to the chassis, enabling it to swim through fluid. | |
 |
|
| 3D microbot chassis a) Schematic representation of a self assembled bio-hybrid microbot with E. coli bacteria appearing in green. b) SEM image of the 3D chassis with a zoomed view of the yellow box. c) Fluorescent bacteria (green channel) are superimposed on a bright-field image of the chassis to show that all microchambers are occupied. Bacteria swimming outside of the microchambers are not permanently embedded in the chassis, but are free to swim away after brief interactions. (Reprinted with permission by Wiley-VCH Verlag) | |
| Compared to biohybrid designs that extract cells from larger organisms, bacteria provide a simpler and more accessible biological component. The microbots can be fabricated by adding an E. coli suspension and allowing single cells to self-load into the chambers. The researchers also genetically engineered the E. coli to express proteorhodopsin, a light-sensitive protein that allows wireless speed control using only green light patterns. | |
| This bioinspired approach allows precise engineering of structures and interfaces between biological and synthetic components. By continuing to optimize biohybrid microbot design and control systems, researchers aim to create sophisticated miniaturized robots able to sense, think, and act at the cellular level. | |
| The microbots designed by physicists at Sapienza University of Rome consist of a tiny 3D-printed chassis equipped with chambers that “trap” individual E. coli bacteria. The trapped bacteria align with the chassis and propel it forward using their natural flagella that they employ for swimming. Each E. coli cell has multiple flagella extending out that can rotate like tiny propellers. The flagella are powered by nanoscale rotary motors embedded in the cell membrane. | |
| The rotational speed of the flagella, and hence the propulsive force they generate, depends on the proton motive force across the cell membrane. By expressing proteorhodopsin, a light-driven proton pump, the researchers gained wireless control over this force. Green light activates the proton pumps, increasing the proton gradient which powers faster flagella rotation and making the bacteria - and their microbot vehicles - speed up. | |
| Two bacteria-driven microbots independently programmed to move from one target to another. | |
| Using motile bacteria as propulsive engines has some key advantages: | |
|
|
|
| By leveraging these natural bacterial behaviors, the biohybrid approach enables consistent propulsion, steering, and wireless control of the microbots. Miniaturization to sub-micron sizes could ultimately be possible by harnessing even smaller bacteria or isolated flagellar motors. | |
| This light-based propulsion system is remarkably efficient, the researchers report. They calculate that the microbots can convert over 3 x 10-11 of the input optical power into thrust – about ten million times more efficient than microscale machines driven by optical tweezers. This high efficiency means that only a few milliwatts of light are needed in total to control hundreds or even thousands of the microbots simultaneously. | |
| The study demonstrates independent steering of multiple microbots along predefined trajectories using patterns of green light tailored to each unit. A central computer analyzes images to determine the position and orientation of each microbot and calculates what adjustments are needed to guide them to the next target. For example, if a microbot needs a counterclockwise nudge to line up with the destination, the computer illuminates the left engine. When it gets close, the trajectory stabilizes into a tight orbit around the target. | |
| While the current study involved navigation along a simple 3-target course, the authors suggest their light-based microbot control system could enable more complex collaborative tasks. The ability to guide each unit independently could allow “swarms” of the tiny robots to distribute themselves and adaptively cover an area, similar to behaviors seen in natural microorganisms. The researchers also propose medical applications, such as using swarms of microbots to perform local scans inside the body or deliver drugs to diseased tissue. | |
| The microbot chassis structures proved key to producing consistent robotic behavior, the researchers emphasize. Earlier efforts to steering swimming bacteria by attaching them to microparticles often struggled with variability in propulsive force. The 3D-printed eight-chamber design enables precise positioning of single motile bacteria. The study results show this repeatable configuration leads to smooth, predictable microbot trajectories. | |
| While still a prototype system, the microbots highlight bacteria as tantalizing candidates for microscale biohybrid robots. Beyond their efficiency, the study authors say bacteria offer a huge diversity of natural sensing, motility, signaling, and feedback behaviors waiting to be incorporated into synthetic designs. By continuing to elucidate and reprogram these living capabilities, they believe biohybrid robots could one day provide a revolutionary toolset for microrobotic medicine, advanced manufacturing techniques, and environmental remediation applications. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
