| Oct 09, 2023 | |
Innovative 3D printing merges electrical cues and shape memory for regenerating bone |
|
| (Nanowerk Spotlight) Millions suffer from bone defects worldwide, caused by disease, injury or age-related conditions. Traditional treatment options include bone grafting, where bone tissue is transplanted from elsewhere in the patient's body or from donors. Autografts from the patient's own body provide the best results but limited availability. Allografts using donated bone avoid supply limitations but risk immune rejection. Such conventional grafting methods have significant downsides, fueling the search for novel tissue engineering solutions. | |
| Advances in 3D printing now allow rapidly customizing porous scaffolds for guiding bone regeneration in irregular defects. However, merely mimicking bone's porous structure is insufficient for robust healing. Bone possesses a property called piezoelectricity, which means it generates small electrical currents when mechanically strained or compressed. Integrating components with similar electrically active piezoelectric properties into scaffolds could provide cues boosting bone tissue regeneration. | |
| Recently, scientists from Wuhan University of Technology developed 3D-printed piezoelectric scaffolds with shape memory using a novel polymer composite ink. By optimizing the piezoelectric nanoparticle filler and print parameters, the scaffolds closely matched bone's electrical properties and mechanics while recovering their structure when heated. Tests showed the scaffolds stimulated stem cell differentiation and significantly improved bone regeneration in rodent skull defects compared to controls. | |
| The research has been published in Small ("3D-Printed Piezoelectric Scaffolds with Shape Memory Polymer for Bone Regeneration"). | |
 |
|
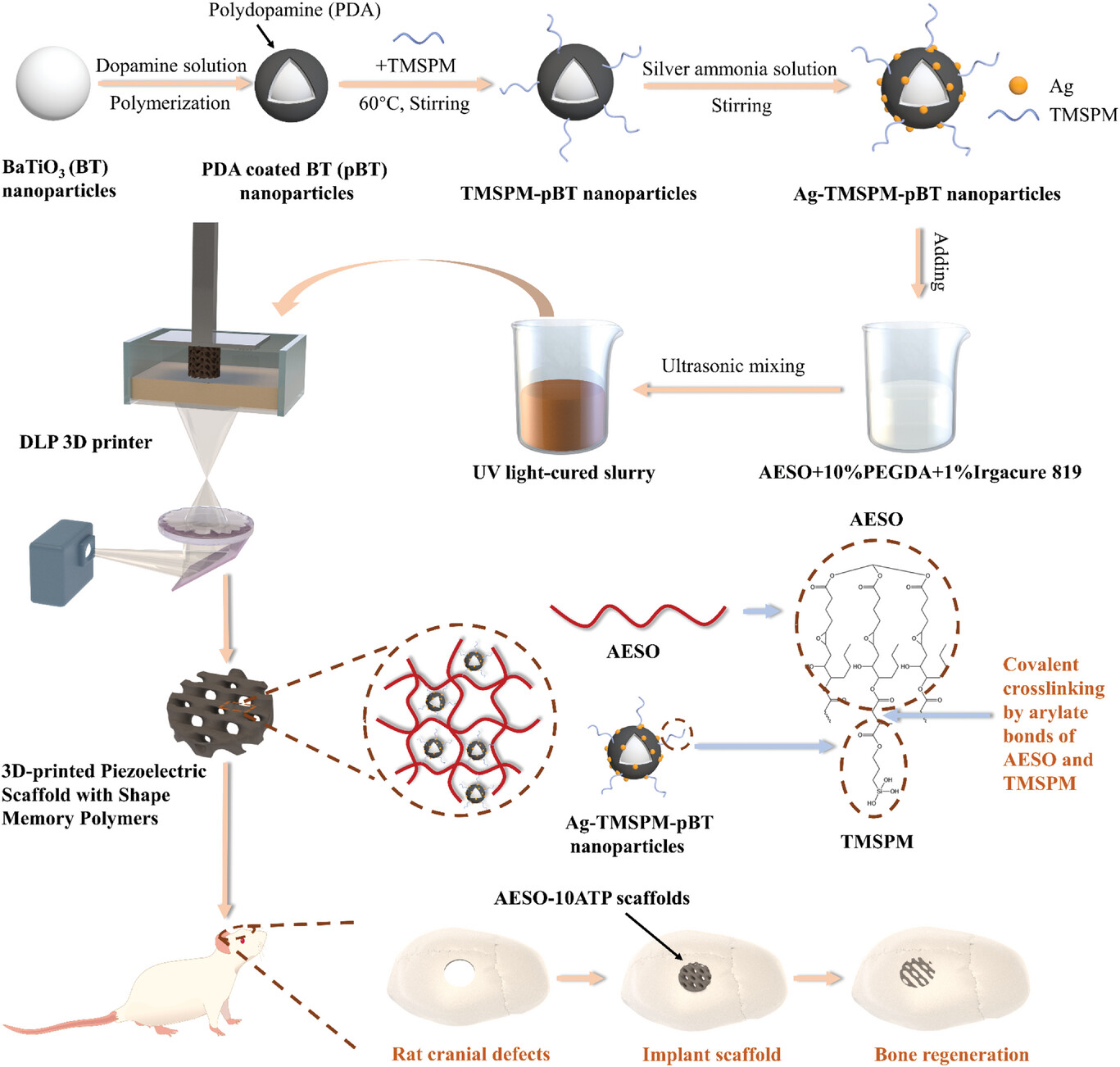
| The preparation process of the Ag-TMSPM-pBT nanoparticles and the AESO scaffolds doped with 10% Ag-TMSPM-pBT nanoparticles (AESO-10ATP scaffolds), and application of the AESO-10ATP scaffolds in bone regeneration. The zoom-in shows the TMSPM from the surface of the Ag-TMSPMpBT nanoparticle and cross-linked with the AESO matrix. (Reprinted with permission from Wiley-VCH Verlag) (click on image to enlarge) | |
| Bones naturally generate tiny electrical currents when stressed that are crucial signals regulating cell growth and repair. Hence, electrical stimulation therapies can accelerate healing bone fractures and defects receiving grafts. However, current approaches like implanted electrodes or external capacitive coupling have limitations. Self-powered piezoelectric scaffolds eliminating such drawbacks have shown promise, but composites preserving strong electrical properties after 3D printing remained challenging. | |
| The researchers leveraged 3D printing and nanomaterial engineering to create enhanced piezoelectric scaffolds for regenerating even complex bone defects. As the printing ink base, they used a biocompatible shape memory polymer called acrylate epoxidized soybean oil (AESO) that can recover its shape when heated temporarily for implantation in narrow sites. To achieve high piezoelectricity, standard filler particles like barium titanate were chemically modified. | |
| First, coating barium titanate nanoparticles with a dopamine polymer layer allowed attaching molecules for crosslinking with the AESO matrix. Further depositing conductive silver nanoparticles enabled polarizing the composite to activate the filler's piezoelectricity. Adding just 10% of modified nanoparticles to the AESO ink gave scaffolds with electrical properties rivalling bone tissue without compromising printability, mechanics and cytocompatibility. | |
| The team demonstrated the merits of their scaffold design with detailed characterization and biological tests. Microscopy and spectroscopy confirmed the nanoparticle modifications and printing accuracy down to the micrometer scale. Mechanical testing showed adequate strength and elasticity combined with shape memory triggered by heating. Osteoblast cell experiments verified good biocompatibility and significantly enhanced differentiation and mineralization when scaffolds were electrically stimulated through ultrasound. | |
| Most importantly, animal studies using rat skull defects evidenced substantially improved bone regeneration for the polarized piezoelectric scaffolds over 4-8 weeks. MicroCT scans revealed considerably higher bone formation that nearly bridged 5-mm wide holes after 2 months compared to both passive scaffolds and empty defects. Histological analysis further confirmed thicker new bone matrix deposition with tighter integration between scaffold and native bone. | |
| The carefully designed piezoelectric composite ink enabled successfully integrating electrical cues into 3D-printed shape memory scaffolds, greatly improving their performance regenerating challenging bone defects in small animals. The simple polarized nanoparticles also avoided complications like electrodes. With customizability and bioactivity exceeding traditional implants, these smart scaffolds could become promising clinical solutions for various orthopedic regeneration needs pending further translational research. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
