| Nov 01, 2023 | |
Scientists engineer carbon nanotubes for greener hydrogen peroxide production |
|
| (Nanowerk Spotlight) Hydrogen peroxide is commonly used for cleaning and bleaching as a friendlier alternative to harsh chemicals. However, the current method for making it isn't that eco-friendly. The process, called anthraquinone oxidation, uses a lot of energy and heat and produces waste. | |
| Scientists are looking for a more sustainable way to make hydrogen peroxide by using electricity, oxygen, and water. In this electric-based method, they use a special coated surface called a "catalyst-coated electrode" to change oxygen into hydrogen peroxide. Oxygen can be changed in different ways, and one way changes it all the way into water. However, for making hydrogen peroxide, the change needs to be stopped halfway, using what scientists call a "two-electron pathway." | |
| Finding the right catalyst that can control this halfway change is crucial, but it has been challenging. If they can figure it out, it would make producing hydrogen peroxide much greener and more efficient. | |
| In a new study published in Advanced Materials ("Tailoring the Atomic-Local Environment of Carbon Nanotube Tips for Selective H2O2 Electrosynthesis at High Current Densities"), researchers from Beijing University of Chemical Technology developed a technique to modify carbon nanotubes (CNTs) to act as highly efficient two-electron oxygen reduction catalysts for electrochemical H2O2 production. | |
| By combining modeling and experiments, they optimized the atomic-scale environment at the tips of broken CNTs, demonstrating a nanomaterial that enables greener, more energy-efficient H2O2 synthesis. | |
 |
|
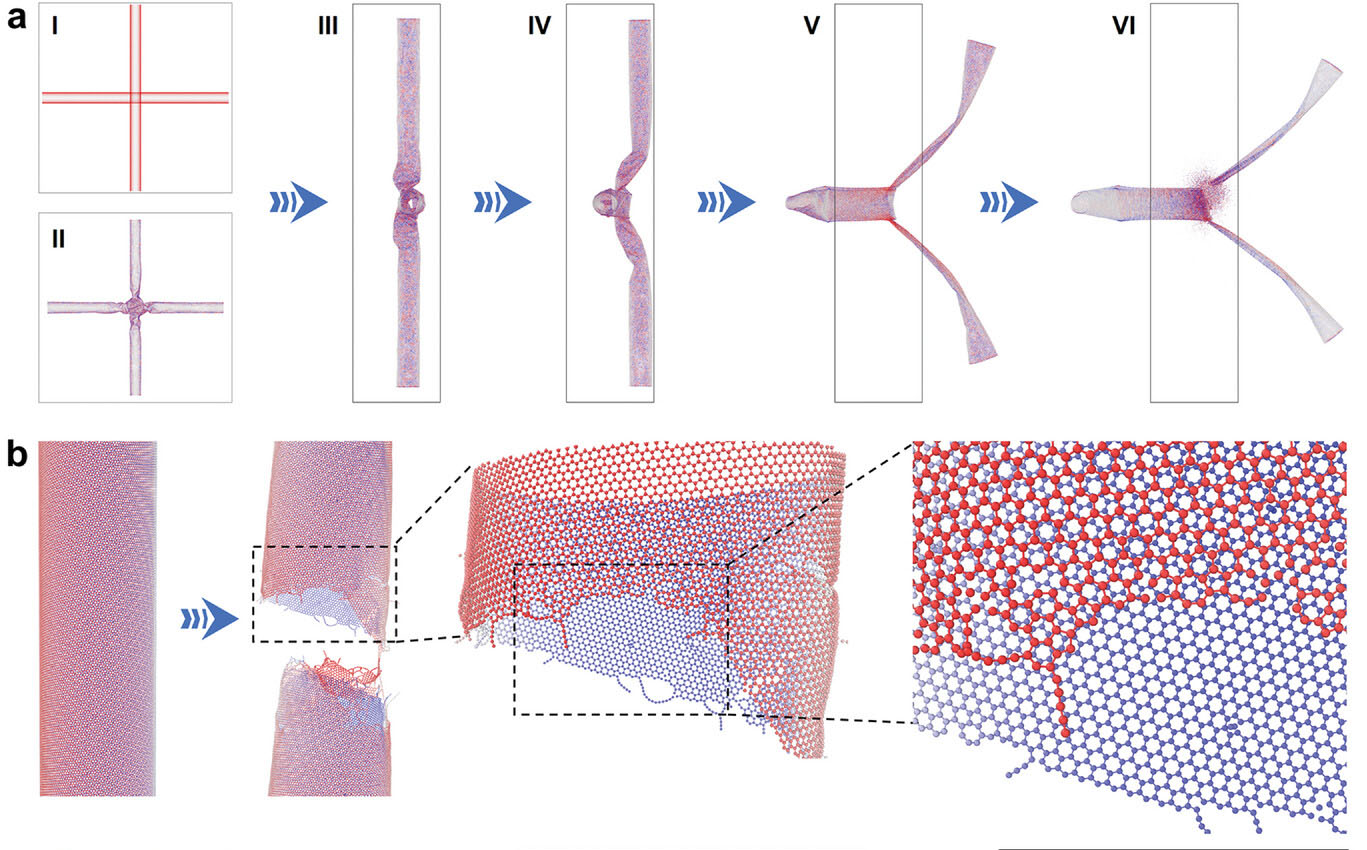
| Cleavage mechanism and tip active sites of CNTs. a) Schematic diagram of initial (I), deformation (II–V), and fracture (VI) state on two entangled CNTs under stretching. I-II are a front view and III–VI are a side view. The darker the color, the higher the shear stress in II–VI. b) Configuration of (112, 28) CNT before and after fracture, and the enlarged tip morphology. (Reprinted with permission by Wiley-VCH Verlag) | |
| The unique structure of CNTs - tiny cylinders of rolled up graphene sheets - gives them advantageous properties for electrocatalysis. But inferior activity and selectivity for the two-electron oxygen reaction has limited their potential. The scientists used simulations and data to reveal that ball milling causes CNTs to preferentially break at junctions between overlapping nanotubes, creating an abundance of open-ended CNT pieces with dangling bonds at the edges that can be doped with other elements. | |
| Density functional theory calculations suggested that adding nitrogen and sulfur to the tips of the carbon nanotubes could help create hydrogen peroxide more effectively, following the 'two-electron pathway' that scientists aim for. This was achieved experimentally by ball milling CNTs with a sulfur precursor, resulting in dual-doped CNTs with nitrogen and sulfur concentrated at the tips according to microscopy and spectroscopy data. | |
| Testing showed the dual-doped tipped CNTs had exceptional performance for selective H2O2 production. They achieved 94.5% H2O2 selectivity at 0.56 V compared to a reversible hydrogen electrode, outperforming other metal-free carbon catalysts. Remarkably, they maintained over 90% selectivity across a 0.59 V potential range – the widest ever reported for this emerging class of electrocatalysts. Using air rather than pure oxygen, the catalyst enabled an unprecedented 30 moles of H2O2 produced per gram per hour at 350 mA/cm2 current density. | |
| The researchers also showed the potential for real-world application by incorporating the CNT catalyst into different electrochemical cell configurations. In an air-based flow cell, the catalyst sustained over 90% efficiency for the two-electron reaction at current densities up to 350 mA/cm2, with negligible performance degradation over 200 continuous hours. Most significantly, they developed a solid-electrolyte cell that uses the catalyst to directly generate pure hydrogen peroxide solution from only air and water. | |
| This research provides a proof-of-concept for using computational modeling to guide design of optimized nanoscale catalysts. The study opens the door to overcoming limitations of carbon-based materials for selective electrochemical production of chemicals like hydrogen peroxide. Looking forward, further development of such customized nanocatalysts promises more sustainable, energy-efficient technologies for on-site, on-demand manufacturing of essential chemicals and fuels. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
