| Nov 06, 2023 | |
Liquid metal skins turned into power source for stretchable batteries and devices |
|
| (Nanowerk Spotlight) Liquid metals are exceptional alloys with melting points so low that they can remain liquid at temperatures close to what we experience in our everyday environment. A popular example in scientific and technological circles is the gallium-indium alloy (GaIn), known for its potential in crafting electronics that bend and stretch with ease. | |
| Yet, one of their characteristics – a tendency to form an oxide layer upon contact with air – has long been a challenge. This "skin" that forms on their surface has generally been considered an obstacle, complicating their integration into practical devices. | |
| But a recent study by Boston University has shifted this perspective. Published in the journal Advanced Functional Materials ("Mechanically Rupturing Liquid Metal Oxide Induces Electrochemical Energy"), the research unveils a novel approach to utilize the electrochemical energy released during the natural reformation of this oxide layer after it's disturbed. This innovative method could pave the way for a new class of soft, flexible batteries and self-sustaining electronics, opening a door to potential applications that were once deemed impractical. | |
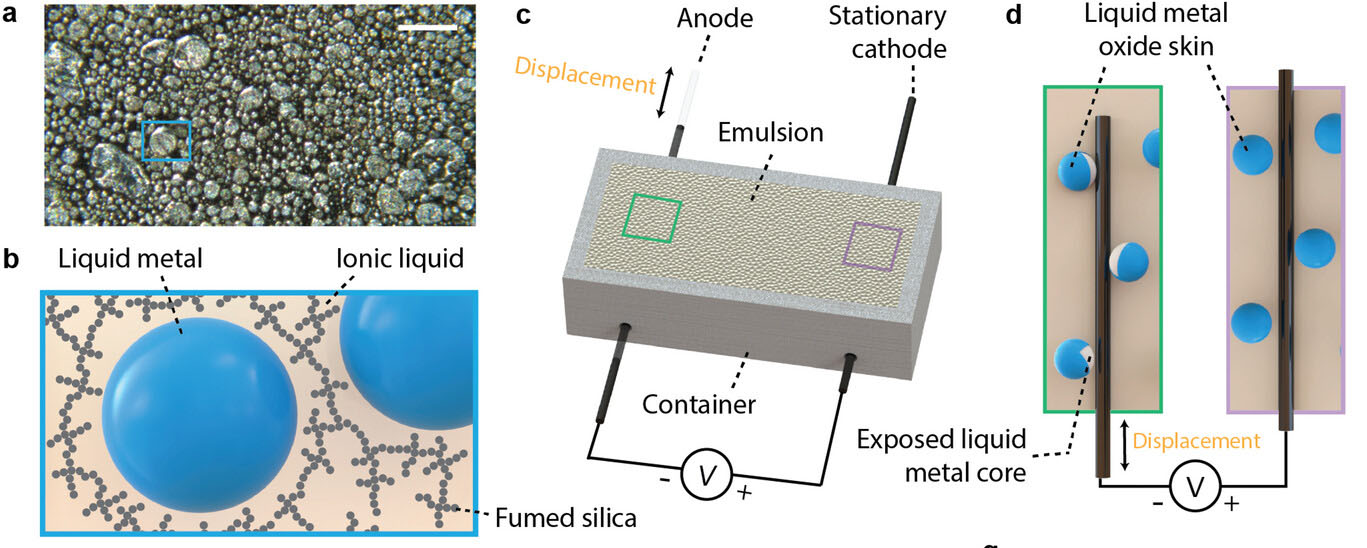
 |
|
| Liquid metal-ionic liquid emulsion. a) Microscope image of emulsion with 90 vol.% of liquid metal. Scale bar represents 100 𝜇m. b) Liquid metal droplets are dispersed in ionic liquid phase and stabilized by fumed silica. c) Relative movement of a rigid wire inside the emulsion induces electrochemcial energy. d) Moving a wire inside the emulsion collides with liquid metal droplets and ruptures their oxide skins. (Reprinted with permission by Wiley-VCH Verlag) | |
| When exposed to oxygen, the surface of liquid metals becomes covered with a thin layer of oxide. This oxide skin protects the underlying metal but is brittle and can be temporarily broken by mechanical forces. The energy released as the oxide layer quickly re-forms is normally wasted. But the Boston University scientists found a way to capture some of that energy and convert it into electrical power. | |
| The team designed a simple cell with two liquid metal drops separated by an ionically conductive liquid electrolyte. As they scratched the surface of one of the drops, breaking its oxide layer, the chemical energy from the re-oxidation reaction generated voltage and current in the cell. Each time the needle moved across the liquid metal, rupturing the oxide skin, they observed voltage and current spikes. No power was produced when the oxide layer was removed using a strongly basic solution, confirming that reforming the oxide was key to generating electricity. | |
| The researchers then created a composite material by dispersing liquid metal droplets stabilized with nanoparticles into the ionic liquid electrolyte. Pulling a wire through this metal-electrolyte emulsion also ruptured the oxide layers, inducing voltage and current. The open circuit voltage reached over 500 mV, and scratching faster or with larger strain increased the output. | |
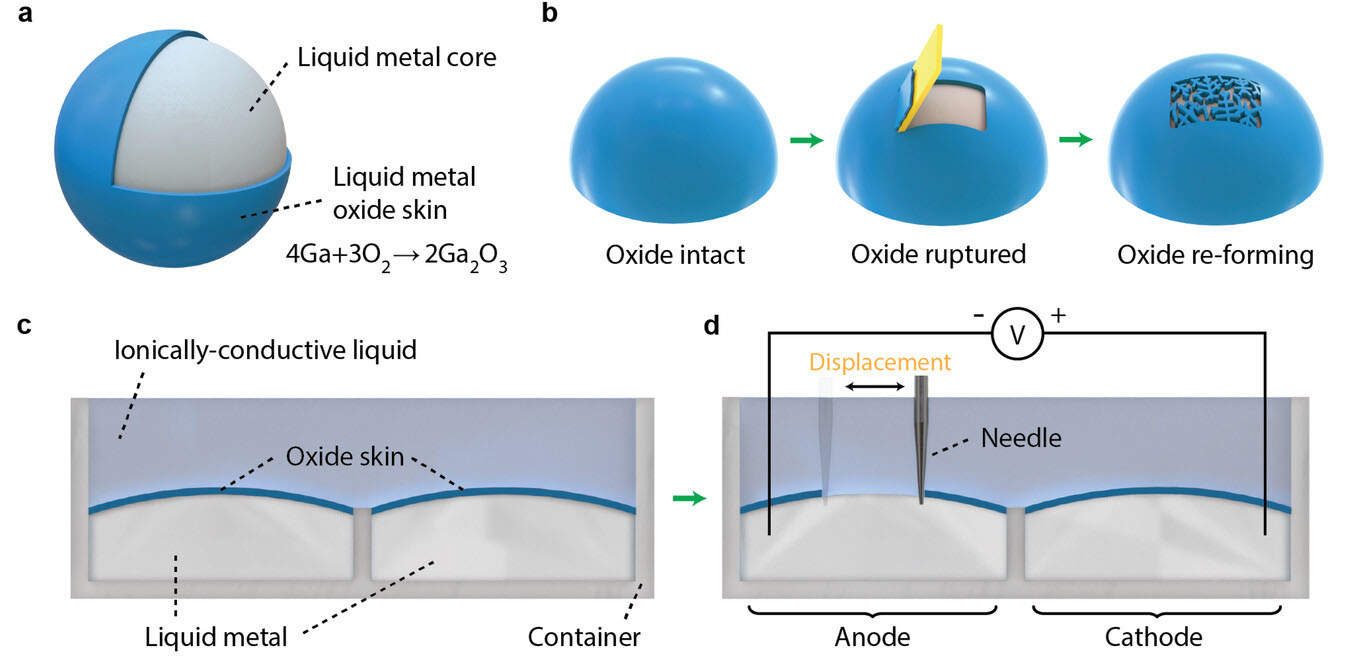
 |
|
| Rupturing liquid metal oxide converts chemical energy to electrical energy. a) The surface of liquid metal is covered by a layer of oxide skin because gallium reacts with oxygen in the environment. b) The liquid metal oxide skin is brittle and can be fractured by mechanical forces, but it quickly re-forms to protect the exposed liquid metal core. c,d) Experimental setup of scratching liquid metal surface with a needle. c) Liquid metal droplets were injected into two separate chambers in a custom container and were immersed in an ionically-conductive liquid electrolyte. d) A needle repeatedly scratched the surface of the liquid metal in one of the chambers to break its oxide skin. The chemical energy of its re-oxidation reaction is partially converted to electrical energy. (Reprinted with permission by Wiley-VCH Verlag) | |
| Encapsulating the emulsion in soft silicone rubber allowed building fully stretchable devices. A serpentine wire embedded in the liquid acted as both electrode and mechanical agitator. Stretching the rubber and wire disrupted the liquid metal droplets, enabling the battery to deliver an open circuit voltage around 500 mV. The device produced a peak power of 4 microwatts, similar to the density of triboelectric nanogenerators. | |
| Importantly, the liquid metal oxide skin shields the underlying metal, giving this soft battery excellent shelf-life stability. The device functioned well after exposure to harsh conditions like high temperatures and immersion in water that normally degrade battery performance. Cycling the battery 20,000 times only moderately decreased the voltage output. | |
| While each cell generates low voltage, the researchers connected eight in series to light an LED. They also built a self-powered keypad that produced voltage spikes when its soft buttons were pressed, demonstrating potential as a sensor. Given the simplicity and customizability of the design, the technology could assist developing stretchable, mechanically activated electronics. Strategies like optimizing the electrolyte conductivity or using metals that oxidize more readily than gallium could further enhance performance. | |
| These findings reveal new possibilities for liquid metal oxide long dismissed as a nuisance in electronics applications. The passive oxide layer both enables capturing the electrochemical energy and provides critical stability. With creative design, the scientists transformed an annoying property into an asset. The liquid metal battery’s abilities to activate on demand and perform in demanding environments position it well for powering future soft robots, wearable devices, and other stretchable technologies. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
