| Jan 05, 2024 | |
Microscopic ion trackers herald new era for organelle communication research |
|
| (Nanowerk Spotlight) Scientists have long sought to decode the complex communication between cellular organelles that regulates virtually all aspects of human health and disease. Mitochondria and lysosomes interact through transient “contact sites”, exchanging vital ions and molecules that control processes from aging to cancer. Yet the nanoscale dimensions and ephemeral nature of these sites have thwarted understanding of how substances flow between organelles, hampering efforts to manipulate this crosstalk for therapeutic ends. | |
| Now, as reported in a recent paper in Small Structures ("Ion Monitoring at Nanoscale Sites of Interorganelle Membrane Contact in Living Cells"), researchers have combined super-resolution microscopy and purpose-built ion biosensors to track pH changes between mitochondria and lysosomes during their microsecond contacts. The work unveils the first high-definition footage of ions flowing between organelles, while demonstrating a platform to screen drugs that modulate this exchange. | |
| The new technique promises to illuminate the longstanding mysteries around interorganelle communication and how it governs cell fate. This could enable unprecedented control over the messaging between cellular compartments for next generation diagnostics and disease treatments. | |
 |
|
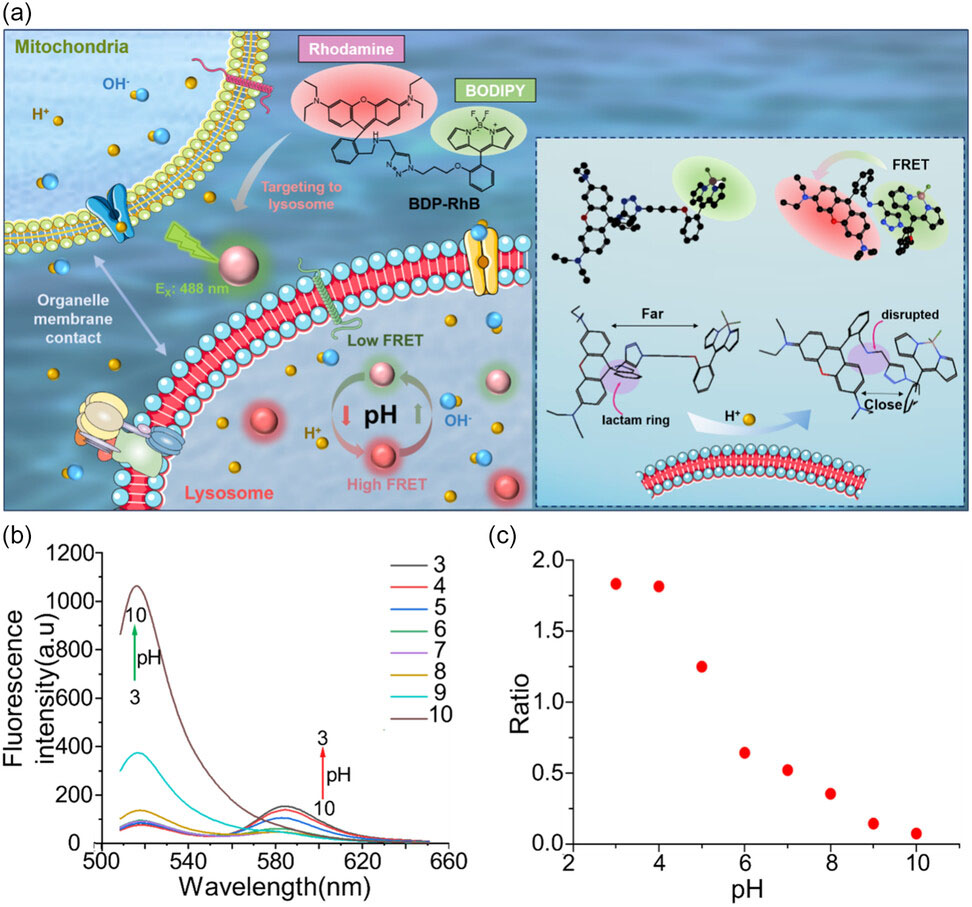
| Design and optical characterization of the H+ responsive probe BDP-RhB. a) Schematic diagram of the design of BDP-RhB for FRET-based fluorescence conversion in lysosomes. b) Fluorescence spectra of probe BDP-RhB (10.0 µM) in solutions with various pH values (3–10). c) The ratio between fluorescence intensity I582/I518 versus pH value. (Reprinted with permission by Wiley-VCH Verlag) | |
Cracking the organelle communication code |
|
| As the body’s power plants which supply energy for cellular processes, mitochondria must closely coordinate with digestive organelles like lysosomes which degrade and recycle biomolecules. The contacts enabling this crosstalk persist for just microseconds to milliseconds, thwarting efforts to capture their signaling dynamics. | |
| Techniques like fluorescence microscopy permit organelle tagging with dyes that switch color in response to ions like pH-sensitive H+. But conventional optical microscopes lack the spatial and temporal resolution to visualize nanoscale contacts and fast transient signals. While super-resolution and high speed microscopes can now resolve organelle boundaries, purpose-built ion detectors have been lacking to track the rapid chemical interplay at these contact points. | |
Joining the dots with specialist ion trackers |
|
| To bridge this gap, a joint research team from China developed an H+ indicator dye that fluoresces green or red depending on pH changes. Conjugating this “FRET” dye to target lysosomes, the group revealed swelling and enhanced acidity in these organelles when contacting mitochondria. This suggests H+ ions may transfer from mitochondria to lysosomes during their microsecond rendezvous. | |
| Notably, the researchers imaged live cells on a “structured illumination microscope” (SIM) which exceeds conventional resolution limits by 2-fold. Capturing 12 frames per second, SIM provided the first nanoscale videos of organelles communicating in living systems. Quantifying changes in fluorescent ratios of the custom pH dye then allowed organelle acidification and inter-organelle crosstalk to be measured for the first time. | |
Screening drugs to intercept organelle messaging |
|
| As well elucidating the basic biology of interorganelle communication, the researchers developed an automated image analysis algorithm to screen drug candidates influencing H+ exchange between mitochondria and lysosomes. | |
| The algorithm classified cellular images based on fluorescent dye ratios and organelle colocalization, rapidly quantifying pH changes and contact states. When screening a panel of autophagy, apoptosis and necrosis pathway drugs, the algorithm successfully stratified compounds expected to increase or decrease organelle acidification. Further machine learning optimization could see such imaging platforms adapted to high throughput drug screening campaigns. | |
Illuminating the role of organelle messaging in disease |
|
| Beyond tools for biomedical research, the work has profound implications for elucidating how organelle contacts control the balance between health and disease. The researchers suggest impaired acidification and elongated mitochondria-lysosome contacts may represent early pathogenic events leading to cancer and neurodegenerative disorders. | |
| While contact points have been observed forming then dissolving between organelles, existing imaging lagged minutes behind the actual signaling events. By capturing ion fluxes with millisecond frames, the new platform promises to expose how organelles communicate to keep cells functioning smoothly; and how errors in this coded exchange triggers catastrophe. | |
| As well as filming these elusive contacts in action, the ability to pharmacologically manipulate crosstalk could spur new generations of ion modulating drugs - conceived by organelle messaging rather than crudely targeting single proteins. | |
| Much further work remains to decode the dialects deployed at different organelle communication hubs throughout the cell. But by resolving the nanoscopic envoys that mediate this traffic, pioneering technologies like this are lighting the way. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
