| Jan 15, 2024 | |
Tailored nanograins enable greener ammonia production |
|
| (Nanowerk Spotlight) Ammonia is indispensable for producing the fertilizers and chemicals that sustain food and material production for billions globally. However, conventional industrial scale ammonia synthesis comes at great environmental costs. The predominant century-old Haber-Bosch method accounts for over 2% of energy use worldwide, while generating substantial carbon emissions. | |
| As global ammonia demand continues rising, there is heightened impetus to develop alternative green production routes with improved energy efficiency and sustainability. | |
| Previous attempts at electrochemically extracting ammonia from air have confronted major roadblocks like poor yields, low efficiency, and lack of stability. Molecular nitrogen (N2) bonds require tremendous energy input to break and activate. Most catalysts fail to adsorb or crack N2 molecules effectively before competing side reactions dominate. Transition metals demonstrate potential, but their conductivity often limits performance. | |
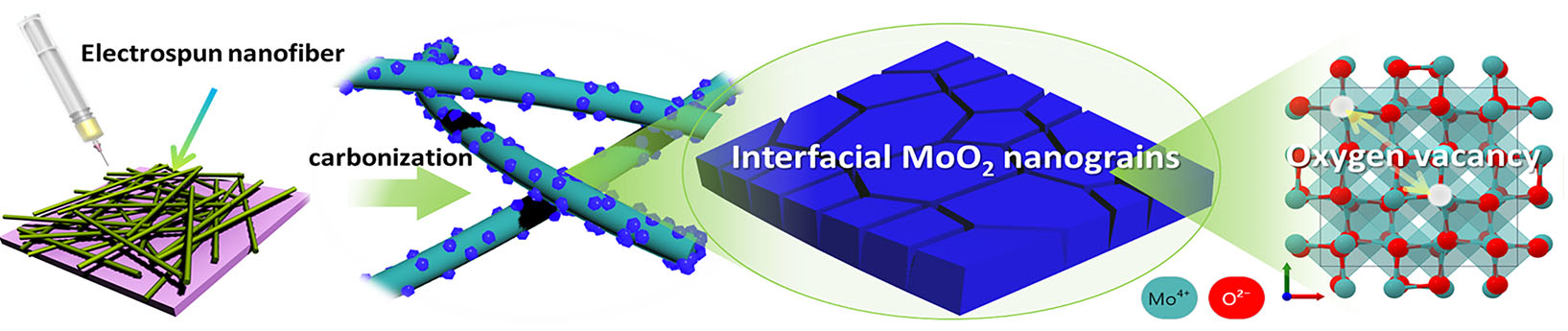
| Seeking to overcome these hurdles, a team of researchers tailored an electrocatalyst consisting of ultrafine molybdenum oxide (MoO2) nanograins anchored throughout nitrogen-doped carbon fiber supports. | |
| “Revolutionizing ammonia production, our research pioneers an eco-friendly alternative to the conventional Haber–Bosch process, mitigating negative environmental impacts and embracing the potential for a greener industrial and agricultural future,” Edison H. Ang, an Assistant Professor at Nanyang Technological University Singapore, tells Nanowerk. | |
| The team’s findings have been published in PNAS ("Optimizing oxygen vacancies through grain boundary engineering to enhance electrocatalytic nitrogen reduction"). | |
 |
|
| Schematic representation of the synthesis of MoO2/Cx. (Reprinted with permission from PNAS) | |
| Their optimization process focused on manipulating MoO2 crystal boundaries to generate abundant oxygen vacancies within the structures. These vacancy sites can strongly trap N2 molecules and provide reactive interfaces to cleave their bonds with less energy, drastically improving reaction kinetics. Meanwhile, the surrounding conductive carbon matrix enhances electron transport to molybdenum centers and prevents aggregation. | |
| “Careful heating of the MoO2/carbon catalyst to 700 °C (MoO2/C700) during preparation proved optimal for producing tiny, interfaced grains with maximized vacancies,” Ang explains. “When we tested it for electrolytic ammonia synthesis in alkaline conditions, this thermally activated material achieved record-breaking rates over four times higher than recently reported metal-based counterparts, converting over a quarter of passed charge into NH3.” He adds that the robust catalyst sustained this high-efficiency ammonia production steadily for 60 hours. | |
| Insight into the underlying mechanisms comes from spectroscopic analyses before and after reacting the optimized MoO2/C700 nanostructures with nitrogen. The data implies N2 molecules initially bind strongly with oxygen vacancy sites, facilitating the crucial transfer of electrons from adjacent molybdenum and carbon to weaken and break the nitrogen-nitrogen triple bond. Further incoming protons then attach to the dissociated atomic nitrogen, ultimately forming ammonia products that remain adhered until increasing buildup. | |
| Advanced simulations corroborate this proposed reaction pathway, revealing over two times stronger nitrogen absorption on oxygen vacancy-laden molybdenum oxide than regular oxide surfaces. | |
| “Subsequent energetics also become more favorable for hydrogenation steps, specifically via an alternate route that proceeds through a key N2H2 intermediate,” Ang points out. “Our computations thus verify observations that vacant defect sites substantial lower barriers in the catalytic process.” | |
| He sums up the team’s results: “When we compared our MoO2/C700 catalyst under the same electrochemical conditions, the combination of specially designed gaps in the material and the carbon-supported nanostructure shows a significant improvement in ammonia production, especially when measured against pure carbon fibers and standard commercial molybdenum oxide.” | |
| While requiring further optimization for industrial implementation, these findings provide proof-of-concept for a more sustainable electrocatalytic production technique to obtain essential ammonia chemicals. The facile and inexpensive nature of the high-performance catalyst synthesis developed here could allow scaling up environmentally benign processes to displace conventional energy-hungry and polluting industrial procedures. | |
| Looking beyond this initial demonstration, the conceptual framework of deliberately engineering oxygen defects in nanostructured composites could aid designing improved catalysts for other challenging reactions like nitrogen reduction. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
