| Feb 25, 2024 | |
Interface-engineered molybdenum oxide catalyst significantly boosts sustainable amine synthesis |
|
| (Nanowerk Spotlight) Secondary amines are an indispensable category of chemicals with extensive applications across industries such as pharmaceuticals, agrochemicals, and polymers. As the demand for amines continues to grow, researchers have been investigating efficient and sustainable methods to synthesize these compounds. | |
| A particularly promising approach is through reductive C-N coupling of nitro compounds, which avoids the need for an extra reduction step typically required when synthesizing amines from nitro compounds. Reductive coupling has gained interest due to its high selectivity and environmental friendliness compared to traditional amine synthesis methods. | |
| Reductive C-N coupling of nitro compounds is a promising approach for sustainable amine synthesis. Transition metal catalysts like molybdenum have shown potential to facilitate this reaction. However, insufficient activity and poor stability remain major challenges limiting practical application. The atomic structure and coordination environment of molybdenum oxide active sites play a key role in determining coupling efficiency. | |
| Seeking to overcome current limitations, researchers have now developed an innovative strategy to substantially improve molybdenum oxide's catalytic performance. Their approach engineers the interface between molybdenum oxide and a carbon layer at the nanoscale to enhance activity and stability. | |
| Published in the journal Chemical Communications ("Enhancing reductive C–N coupling of nitrocompounds through interfacial engineering of MoO2 in thin carbon layers"), the study details the creation of a silica-supported molybdenum oxide catalyst coated with a thin defective carbon layer. | |
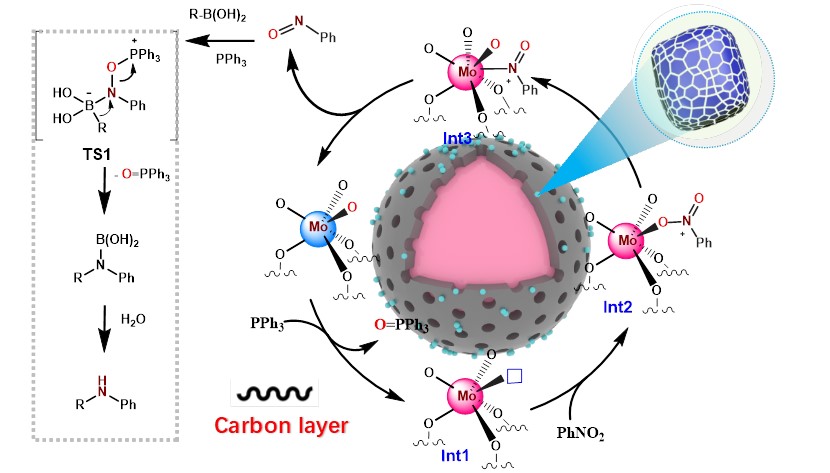 |
|
| Graphical abstract of this work depicting the catalytic pathways of amine synthesis. (Image: Royal Society of Chemistry) | |
| "Our research puts forth an entirely new concept of optimizing metal oxide catalyst performance through engineering the interface between the active metal component and a carbon layer support, Dr. Edison H. Ang, an Assistant Professor at Nanyang Technological University Singapore, tells Nanowerk." | |
| The large number of crystal grain boundaries generated in the carbon-confined MoO2 nanoparticles enables abundant catalytically active sites. In addition, the strong metal-carbon interfacial interactions facilitate electron transfer during the reaction. Tests demonstrate this structurally optimized catalyst achieves excellent activity and stability in reductive C-N coupling of various nitro compounds to synthesize secondary arylamines under mild conditions. | |
Putting this Research in Context |
|
| Amines are prevalent in pharmaceuticals, pesticides, and more. Traditional amine synthesis from nitro precursors requires risky hydrogenation procedures. Direct reductive coupling of nitro compounds with boron compounds enables cleaner, more selective amine production. Molybdenum-based catalysts have shown promise for driving this reaction. However, poor activity and material instability remain key limitations. | |
| Recently, strategies like introducing oxygen into molybdenum disulfide crystals or increasing edge sites in molybdenum oxide have improved reactivity by modulating electronic structure or active surface area. Yet, optimizing the atomic coordination environment and interfacial behavior in nanoscale molybdenum oxide grains could further enhance performance. The current study investigates a novel interface engineering approach to unlock molybdenum oxide's full catalytic potential for reductive coupling. | |
Optimizing Interfacial Sites in Molybdenum Oxide Nanograins |
|
| In this work, the researchers coated silica nanoparticles with molybdenum precursor using polydopamine polymerization. Heating transformed the polymer layer into thin defective carbon coating the silica while molybdenum oxide nanoparticles formed inside. Tests of this catalyst, Mo/C@SiO2, revealed abundant MoO2 crystal interfaces confined within the carbon, in contrast to conventional MoO2 synthesized under air oxidation. | |
| The team systematically optimized synthetic conditions like molybdenum loading and carbonization temperature to achieve maximal grain boundary density and carbon-metal interconnectivity. Advanced electron microscopy and spectroscopy characterization confirmed successful incorporation of tiny MoO2 grains in the defective carbon layer interacting strongly with the metal. The interface engineering strategy generated a large active surface area and facilitated redox electron transfer during catalysis. | |
| "The large number of molybdenum oxide grain boundaries generated by confining nanoparticles in the defective carbon coating resulted in abundant catalytically active sites and significantly boosted reductive coupling capability, explains Ang." | |
Significantly Enhanced Reductive Coupling Performance |
|
| Mo/C@SiO2 demonstrated excellent activity, selectivity, and stability for reductive C-N coupling of various aryl nitro compounds with aryl boronic acids to synthesize functionalized secondary arylamines. The team achieved a 94% yield of the model product p-methoxy-N-(4-nitrophenyl)aniline under mild 100 °C temperature using toluene solvent. | |
| The optimized Mo/C@SiO2 catalyst exhibited substantially higher yields compared to conventional MoO2 and even outperformed an analogous catalyst prepared under air oxidation rather than carbonization. Electron microscopy, X-ray diffraction, XPS, and other analysis confirmed the critical role of the engineered carbon-metal interface in enhancing MoO2 activity. | |
| Tests also verified Mo/C@SiO2 could couple more complex and aliphatic substrates. The catalyst retained reasonably high reusability over 4 cycles. Post-reaction studies indicated partial molybdenum leaching and carbon layer damage occurred during catalysis, contributing to activity loss. Nonetheless, the carbon coating still stabilized MoO2 much better than the catalyst without carbon. | |
Broader Impacts |
|
| This study puts forth a novel and effective strategy to advance molybdenum oxide catalysts for synthesizing valuable amine compounds. The facile interface engineering method significantly improves MoO2's coupling activity and stability through synergistic effects between the carbon layer and optimized metal oxide morphology. | |
| "Considering the practical challenges and environmental impacts of conventional amine synthesis methods, our sustainable catalyst innovation enables greener and more selective production of these vital chemicals by direct coupling of nitro compounds," Ang concludes. "The general concept of using defective carbon layers to optimize metal oxide interfaces could also inform design of catalysts for other reactions. With further development, the robust and highly active interface-engineered molybdenum oxide catalyst may enable more efficient and environmentally responsible production of pharmaceuticals, agrochemicals, and specialty chemicals." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
