| Feb 28, 2024 | |
New tech harvests both magnetic and ultrasound energy to safely power medical implants |
|
| (Nanowerk Spotlight) The promise of implantable medical devices transforming healthcare has long been tempered by practical power limitations. Bulky batteries demand frequent charging or replacement surgery risking complications. These outcomes cause substantial cost, morbidity and frustration while stymying further miniaturization advances. Despite intense R&D, existing wireless power solutions remain inadequate in the face of complex tradeoffs balancing patient safety, transmission efficiency and implant lifespan. | |
| Now, researchers have developed an innovative dual energy harvesting technology potentially overcoming these enduring challenges. By safely combining magnetic field and ultrasonic energy, their novel device generates 300% higher power than the current state-of-the-art devices. This breakthrough may finally provide robust wireless power to usher in smarter, smaller implants. | |
| The researchers published their findings in Energy & Environmental Science ("Magnetic field and ultrasound induced simultaneous wireless energy harvesting"). | |
 |
|
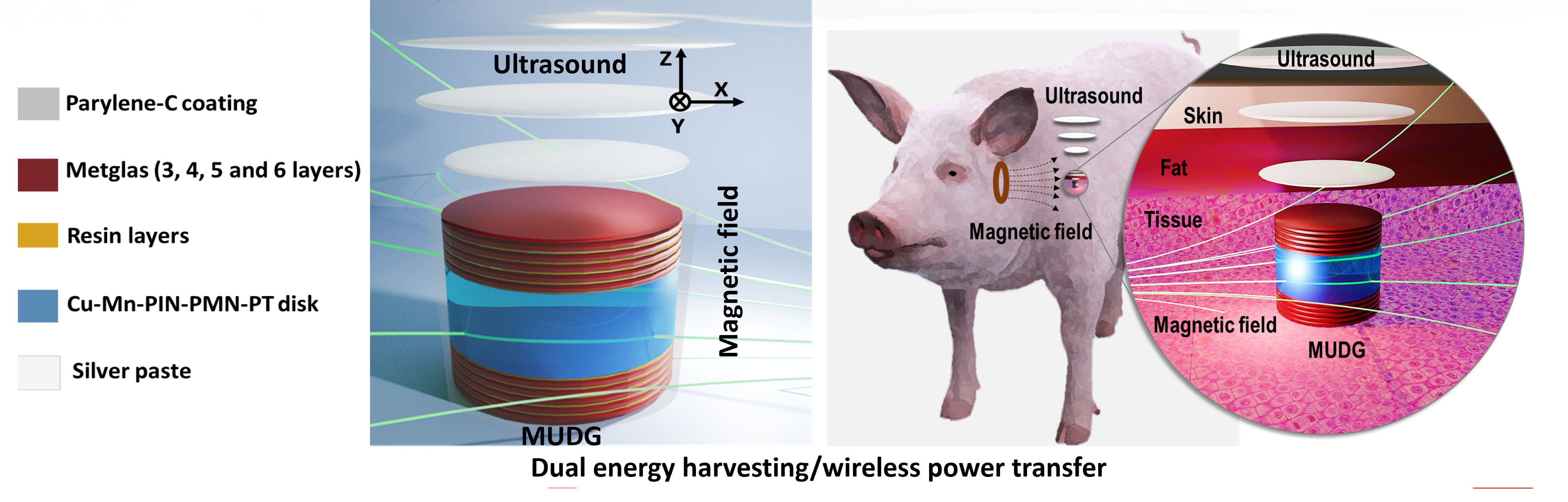
| Graphical abstract of the work. (Image: RSC Publishing) | |
The Power Problem Holding Back Implant Technology |
|
| Traditional approaches to powering implantable medical devices have predominantly relied on inductive coil systems or batteries, each with significant drawbacks. Inductive coils, for instance, suffer from rapid efficiency losses when there is misalignment or an increase in distance between the transmitter and receiver, making them less reliable and limiting the practicability of their use in dynamic bodily environments. | |
| Furthermore, these systems often operate at high frequencies (10 to 100s of MHz) that can lead to excessive absorption by body tissues, necessitating compromises between safety and power delivery. Batteries, on the other hand, introduce challenges related to size, longevity, and the need for surgical replacement, posing risks of infection and other complications. | |
Dual Harvesting: Combining Magnetic and Acoustic Energy As Complementary Power Sources |
|
| “In our new study, we outline the discovery of an innovative dual harvesting system which combines magnetic fields and ultrasound energy,” Dr. Sumanta Kumar Karan, a postdoc researcher at Penn State University, and lead author of the study, tells Nanowerk. “Individually, neither could deliver enough power to safely power up the implants below safety limits. However, in concert their complementary advantages enable major leaps in capability.” | |
| The team fabricated a miniaturized piezoelectric disk fitted with magnetostrictive layers. This novel device can convert simultaneously magnetic fields and acoustic ultrasound wave into electricity via magnetoelectric and piezoelectric effects at low working frequency (∼250 kHz). | |
| Magnetic and ultrasonic energy attenuate far less traversing organic tissue than electromagnetic or optical radiation. The transmitters and implanted receiver can thus maintain adequate intensity for efficient power transfer across greater depths. But perhaps more vitally, the combined harvesting keeps exposure within accepted bio-safety limits for both modalities. Rather than struggling at the ragged edge of intensity caps, the dual approach distributes the power burden across dual underutilized sources. | |
Records Over 50 Milliwatts Output Power in Lab and Tissue Tests |
|
| In air and aqueous testing, the prototype Magneto-Ultrasound Dual Generator (MUDG) achieved an ultra-high output reaching 52.1 mW. No previous wireless methods have produced such levels under comparable conditions. This milestone came at 500 mT magnetic flux exposure and 675 mW/cm2 ultrasonic intensity – both comfortably below IEEE and FDA defined human body regs. Indeed, even assuming much larger transducers, output could scale over 100 mW before nearing restrictions. | |
| “Notably, our results held up impressively even when demonstrating real world viability for implants,” Karan points out. "In ex vivo trials, we embedded prototypes in animal tissue samples up to 22x40 mm and 15 mm thick. Despite the challenging environment, the device still reached over 50 mW – enough to swiftly recharge several batteries with different capacities in short time frame below the safety limit.” | |
| Such performance promises to give implanted devices true perpetual operation without ever opening the patient again. The system could even tweak its balance of acoustic and magnetic inputs to optimize efficiency for a given implant depth. | |
Broad Applications Beyond Healthcare - Powering Sensor Networks and Smart Infrastructure |
|
| Beyond merely powering next gen medical implants, the researchers suggest some other tantalizing potential applications for their invention. The robust wireless power transfer and miniaturized footprint make it an excellent fit for networks of distributed sensors and internet-of-things devices. | |
| These could monitor infrastructure integrity in difficult to access areas, track environmental metrics, or form self-powered smart sensor grids transmitting data without batteries or wiring. The versatility of dual energy harvesting also facilitates flexibility in real world deployment. One input could top up the device depending on which type of ambient source was more abundant in that particular locale. | |
Building Towards Even Smaller and More Capable Implants |
|
| While a major leap ahead, the inventors identify routes to refine the breakthrough even further. Integrating custom acoustic components and piezoelectrics could enhance efficiency. Impedance matching between layers would help minimize signal reflection and loss at each interface. Though highly biocompatible, the polymer encapsulation around prototypes could also employ exotic metamaterials to protect implanted devices from biochemical reactions and detection by the immune system. | |
| Additionally, further miniaturization remains possible by using thinner piezoelectric films and optimized geometries. Indeed, overcoming excessive device bulk has stymied innovation. This platform finally provides scalable power to drive capabilities from stimulating individual nerves to wirelessly controlling organ function – opening up an unexplored vista for capabilities from programmable bio-chips to full cybernetic organs. | |
| With smarter implanted devices no longer just a pipe dream, this new dual harvesting paradigm may presage exciting medical horizons. | |
| The researchers continue enhancing their breakthrough invention and collaborating with partners towards eventual clinical translation. While further confirms are still needed, their innovative solution points toward finally overcoming the power problem impeding implant applications both in and beyond healthcare. Devices once unimaginable may soon become lifesaving and life-changing realities. | |
| Other Penn State researchers contributing were Bed Poudel, research professor, Department of Materials Science and Engineering; Haoyang Leng former doctoral student; Mehdi Kiani associate professor of Electrical Engineering; Sujay Hosur, doctoral candidate and Zeinab Kashani, former PhD student. Kai Wang and Rammohan Sriramdas, former assistant research professors at Penn State and Shashank Priya, vice president for research at the University of Minnesota and former professor at Penn State also contributed. Andrew Patterson, professor in the Department of Veterinary and Biomedical Sciences and Anitha Vijay, research technologist are also contributed. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
