| Mar 13, 2024 | |
Silk peptide nanoparticles show promise for enhancing targeted cancer therapy through dual-drug delivery |
|
| (Nanowerk Spotlight) Cancer remains one of the most challenging diseases to treat, despite decades of research. While targeted therapies like epidermal growth factor receptor (EGFR) inhibitors have shown promise, their effectiveness is often limited by poor bioavailability (the proportion of drug that reaches circulation after administration) and the development of drug resistance in cancer cells. | |
| In recent years, nanoparticle-based drug carriers have emerged as a potential solution to these challenges. These tiny particles, typically less than 200 nanometers in size, can encapsulate drugs and deliver them more efficiently to tumor sites. They can improve drug solubility, prolong circulation time, and even enable targeted delivery to cancer cells while sparing healthy tissue. | |
| Among the various materials explored for nanoparticle fabrication, silk fibroin from silkworm cocoons has garnered interest due to its biocompatibility (not harmful to living tissues), biodegradability (can be broken down naturally), and versatility. Building on this foundation, researchers from Wuhan University and Anhui Medical University in China have developed a unique drug delivery system using silk peptide nanoparticles. | |
| As reported in the journal Nano Select ("Enhancing targeted cancer therapy through multiple drug delivery by silk peptide nanoparticles"), these nanoparticles can simultaneously deliver two drugs – the EGFR inhibitor erlotinib and the natural compound curcumin – resulting in dramatically enhanced anti-cancer effects. This co-delivery approach represents a significant advancement over existing single-drug carriers. | |
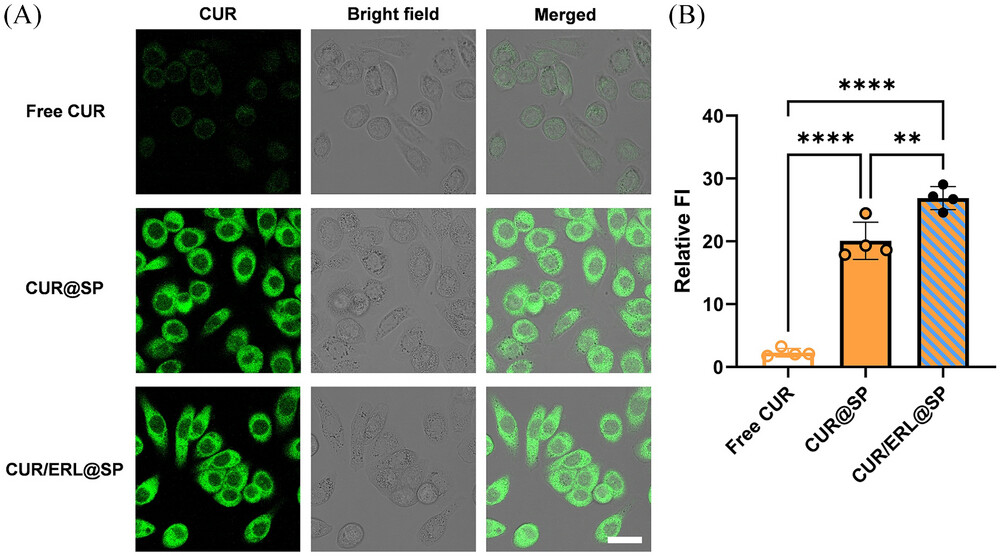 |
|
| Observation on intracellular curcumin by confocal laser scanning microscopy imaging. A, CLSM images of PC-9 cells incubated with the free drug and drug loaded nanoparticles with the CUR concentration of 20 µg mL−1, respectively, for 4 hours. Scale bar: 30 µm. B, Relative fluorescence intensity (FI) of PC-9 cells analyzed by ImageJ v1.8.0 software. PC-9 cells were incubated with the free drug and drug loaded nanoparticles with the CUR concentration of 20 µg mL−1, respectively, for 4 hours. Data are mean ±SD, n = 4. The statistical significance between two sets of data was calculated using Student’s t test. **P <0.01 and ****P <0.0001. | |
| The research team, led by Zi-Hao He and Li-Jin Qi, first extracted silk peptides from silkworm cocoons and fine-tuned the production process to obtain nanoparticles smaller than 150 nanometers. Interestingly, they discovered that curcumin played a crucial role in stabilizing the nanoparticles when co-loaded with erlotinib. This allowed them to overcome past challenges in developing erlotinib nanocarriers with consistent size and good stability. | |
| When tested on lung cancer cells, the dual-drug silk nanoparticles showed remarkable results. They were taken up by the cells much more efficiently compared to the free drugs, leading to a 67% increase in intracellular drug accumulation. This enhanced delivery translated into significantly stronger inhibition of cancer cell growth and increased cell death by apoptosis (programmed cell death). | |
| Investigating the underlying mechanisms, the researchers found that the nanoparticle-mediated co-delivery led to effective suppression of EGFR and its activated form (phosphorylated EGFR), which are key drivers of cancer progression. The treatment also favorably modulated apoptosis-regulating proteins, increasing pro-apoptotic Bax while decreasing anti-apoptotic Bcl-2 and survivin. | |
| Notably, the dual-drug silk nanoparticles demonstrated multi-faceted effects against cancer progression pathways. They hindered the epithelial-mesenchymal transition (a process that allows cancer cells to become more invasive and spread), reduced the expression of immunosuppressive factors like PD-L1 (which help cancers evade immune attack), and inhibited cell fusion-promoting proteins (linked to tumor progression). This suggests the potential for a comprehensive anti-cancer impact. | |
| To validate the clinical relevance of their findings, the team tested their nanoparticles on circulating tumor cells isolated from a lung cancer patient's blood. The erlotinib-curcumin-loaded nanoparticles achieved a striking reduction in EGFR expression in these patient-derived cells, highlighting the translatability of this approach. | |
| While these results are promising, several challenges remain before this technology can reach clinical application. Large-scale manufacturing of the silk peptide nanoparticles needs to be optimized, and rigorous safety and efficacy testing in animal models and human trials will be required. The long-term fate of the nanoparticles in the body and any potential side effects also need to be carefully evaluated. | |
| Despite these hurdles, the innovative work by He, Qi, and colleagues represents a significant stride in nanomedicine for cancer. By leveraging the unique properties of silk peptides and the synergistic potential of dual-drug delivery, their platform opens new possibilities for more effective and targeted cancer therapies with fewer side effects. With further development and validation, this bio-inspired approach could bring us closer to the goal of truly personalized, precise cancer medicines that can transform patient outcomes. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
