| Posted: Aug 22, 2008 | |
Nanotechnology based stem cell therapies for damaged heart muscles |
|
| (Nanowerk Spotlight) Regenerative medicine is an area in which stem cells hold great promise for overcoming the challenge of limited cell sources for tissue repair. Stem cell research is being pursued vigorously in laboratories all over the world (except in the U.S., where federal funding for embryonic stem cell research has been severely restricted by the current administration) in the hope of achieving major medical breakthroughs. Scientists are striving to create therapies that rebuild or replace damaged cells with tissues grown from stem cells and offer hope to people suffering from cancer, diabetes, cardiovascular disease, spinal-cord injuries, and many other disorders. | |
| Embryonic stem cells are pluripotent. That means that during normal embryogenesis – the process by which the embryo is formed and develops – human embryonic stem cells can differentiate into all derivatives of the three primary germ layers: ectoderm, endoderm, and mesoderm. Researchers have also found undifferentiated cells – adult stem cells – in children and adults. Unlike embryonic stem cells, the use of adult stem cells in research and therapy is not controversial because the production of adult stem cells does not require the creation or destruction of an embryo. | |
| Often, adult stem cells are not pluripotent but multipotent. That means they can differentiate only into a limited variety of cell type. One such example are mesenchymal stem cells (MSC) – adult stems cells found in bone marrow which can be differentiated into bone, cartilage, fat, and connective tissues – which offer tremendous potential for the repair and or regeneration of damaged tissues and organs. | |
| An area of particular interest is differentiation of MSC into cardiomyocytes (let's simply call them 'heart muscle cells') for damaged heart muscle tissue. In a heart attack, part of the heart muscle loses its blood supply and cells in that part of the heart die, thereby damaging the muscle. This reduces the ability of the heart to pump blood around the body. Considering that coronary heart disease is the leading cause of death in most Western countries (in America with almost half a million fatalities and well over 1 million new and recurrent coronary attacks), stem cell therapy – to repair heart muscle cells, and restore the viability and function of the area already damaged – could have a tremendous impact on modern medicine. | |
| "Recently, carbon nanotubes (CNTs) have been generating great excitement in the fields of bioengineering and drug delivery research – however, very little is known about the affect of CNTs on MSC response" Dr. Valerie Barron tells Nanowerk. "Therefore, the main aim of one of our recent research studies was to investigate the effect of CNTs on human MSC (hMSC) biocompatibility, proliferation and multipotency." | |
| In this study, Barron, a Senior Researcher at the National Centre for Biomedical Engineering Science at National University of Ireland (NUI), together with collaborators from NUI's Regenerative Medicine Institute and Department of Anatomy, investigated a range of different types of CNTs,including single-walled nanotubes (SWCNTs), multi-walled nanotubes (MWCNTs) and functionalized CNTs. | |
| Reporting their findings in the July 12, 2008 online edition of Nano Letters, ("Carbon Nanotubes and Mesenchymal Stem Cells: Biocompatibility, Proliferation and Differentiation"), first-authored by Barron's colleague Emma Mooney, the NUI, Galway scientists revealed that at low concentrations of COOH-functionalized SWCNTs, the CNTs had no significant effect on cell viability or proliferation. In addition, by fluorescently labeling the COOH functionalized SWCNTs, the CNTs were seen to migrate to a nuclear location within the cell after 24h, without adversely affecting the cellular ultrastructure. Moreover, the CNT had no affect on adipogenesis, chondrogenesis or osteogenesis. | |
 |
|
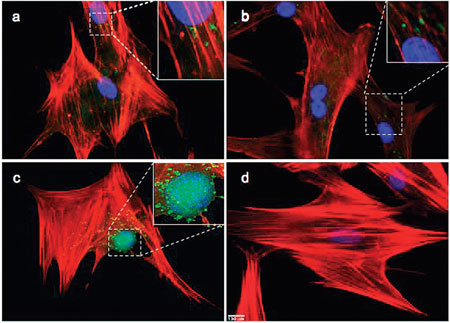
| Uptake of COOH-functionalized SWCNT by the cell. Fluorescent images of biotinylated CNT within the cell after (a) 24 h, (b) 48 h, and (c) 6 days and (d) hMSC alone (scale bar 130 µm). (Reprinted with permission from American Chemical Society) | |
| Previous research has shown that CNTs migrate into cancer cells and therefore can be used for biomolecule delivery directly into the cells. This is the first study to examine the effect of CNTs on hMSC and as such is important for new and emerging technologies in drug delivery, tissue engineering, and regenerative medicine. At low concentrations, CNTs have minimal affect on MSC viability and multipotency. Therefore, they have great potential to advance the field in a number of ways including | |
|
|
|
| In a previous position at Trinity College Dublin, Barron had worked in Werner Blau's Molecular Electronics and Nanotechnology group where she gained a tremendous appreciation for carbon nanotubes. "As a biomaterials scientist, I could see their potential in biomedical applications" she says. At NUI, Galway she therefore teamed up with Murphy to examine the effect of CNTs on MSC differentiation. Both researchers were aware of the fact that, since there is no clinical therapy available for the repair of damaged heart muscle, there exist tremendous opportunities for the creation of novel nanotechnology based therapies. | |
| Since carbon nanotubes are electrically conductive, there is a huge potential for the manipulation of MSC differentiation pathways to create electroactive cells such as those found in the heart. In particular, specific applications could result in novel MSC based cell therapies for electroactive tissue repair; novel biomolecule delivery vehicle for manipulation of MSC differentiation pathways; and electroactive CNT scaffolds for damaged electroactive tissues. | |
| "At present, we are developing a novel electrophysiological environment to promote MSC differentiation towards a cardiomyocyte lineage" says Barron. "In the short-term we plan to focus on optimizing this approach to develop nanotechnology based cell therapies. In the longer term we hope to use the nanotubes as delivery vehicles for a range of different biomolecules for the manipulation of MSC differentiation pathways towards a range of different cell types." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
