| Posted: Sep 04, 2008 | |
Nano road blocks stop molecular traffic and lead to novel nanotechnology detection scheme |
|
| (Nanowerk Spotlight) Borrowing from nature's micro- and nanoscale propulsion systems, nanotechnology researchers have successfully used motor proteins to transport nanosized cargo in molecular sorting and nano-assembly devices (we just addressed aspects of this topic in our Nanowerk Spotlight from a few days ago – Nanotechnology transport systems get a closer look). In so-called gliding assays, surface-attached motors propel cytoskeletal filaments, which in turn transport a cargo. However, cargo and motors both attach to the filament lattice and will affect each other. While an effect of cargo loading on transport speed has been described before, it has never been explained very well. | |
| To study this effect, scientists in Germany have observed single kinesin-1 molecules on streptavidin coated microtubules. They found that individual kinesin-1 motors frequently stopped upon encounters with attached streptavidin molecules. This work helps to understand the interactions of kinesin-1 and obstacles on the microtubule surface. An interesting, possibly even more important side result is that this understanding will not only help to optimize transport assays, balancing speed and cargo-loading, but can be used as a novel method for the detection of proteins as well. | |
| "To understand what happens, we need to look at kinesin-1's motor mechanism" Dr. Stefan Diez explains to Nanowerk. "Even though microtubules are made of ∼13 protofilaments, it has been shown that kinesin-1 follows only one of them during one run and does not 'switch lanes'. Kinesin-1's runs last for around 100 steps. This behavior makes an encounter of kinesin-1 and cargo on the microtubule surface highly likely." | |
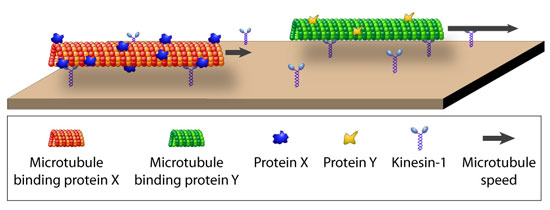 |
|
| The speed of protein-coated microtubules gliding on a kinesin surface is determined by the density of the coating protein and can be used for differential detection. (Image: Diez group, Max Planck Institute of Molecular Cell Biology and Genetics) | |
| While there has been indirect evidence for kinesin-1's stopping at roadblocks, Diez and his group were the first to directly show that kinesin-1 stops when it encounters an obstacle on the microtubule lattice. The Max Planck scientists were able to do this using quantum dots as roadblocks and particle tracking to follow single kinesin molecules stopping when they encounter these quantum dots. | |
| Diez attributes the deceleration of cargo-laden microtubules in gliding assays to an obstruction of kinesin-1 paths on the microtubule lattice rather than to 'frictional' cargo-surface interactions. This can be explained by kinesin’s particular properties: Kinesin moves in a hand-over-hand mechanism along a single protofilament. Its processivity requires the rear head to stay bound until the leading head is firmly attached to the next tubulin dimer along the protofilament. Therefore, says Diez, if a large molecule is blocking the next tubulin dimer, the leading head cannot bind and the rear head cannot detach. This situation effectively stalls the kinesin molecule. | |
| "In a gliding assay, where each microtubule is transported by many kinesin motors, the stalling of some kinesin molecules causes a significantly increased drag force during the period of their attachment and provides an explanation for the slow-down of gliding microtubules." | |
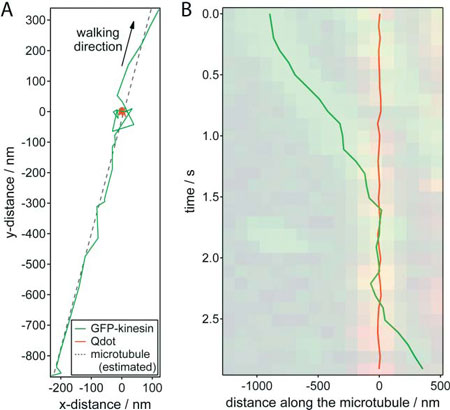 |
|
| Nanometer tracking of a typical encounter between a GFP-kinesin molecule and a quantum dot roadblock. (A) x-y trajectories of the GFP–kinesin molecule (green) and quantum dot (red).The microtubule position was estimated by linear regression of the GFP–kinesin data (dotted line). (B) Distance of the GFP–kinesin molecule and the quantum dot along the microtubule (relative to the average position of the quantum dot) plotted against time. The dual color kymograph, derived from the widefield TIRF imaging was put transparently in the background for comparison. (Reprinted with permission from Royal Society of Chemistry) | |
| Together with his PhD student Till Korten, who first-authored the paper, Diez presents these new findings in Lab on a Chip ("Setting up roadblocks for kinesin-1: mechanism for the selective speed control of cargo carrying microtubules"). | |
| Diez and Korten point out that a very intriguing aspect of their work is that – beyond an improved understanding of motor interactions with cargo-laden microtubules – this obstacle-caused slow-down of gliding microtubules could be applied in a novel molecular detection scheme. The way this would work is that the slow-down of cargo-carrying microtubules can be exploited to detect the binding of proteins to microtubules. Due to the small dimensions of microtubules, this method has the potential to be used for the detection of proteins in single cells. | |
| "Using a mixture of two distinct microtubule populations that each bind a different kind of protein, the presence of these proteins can be detected via speed changes in the respective microtubule populations," says Diez. | |
| Microtubule based transport has already been used to sort and to concentrate transported cargo. Diez and his group envision that these methods could be combined with their detection method to create highly integrated lab-on-a-chip devices. These devices would then be able to detect, sort and concentrate proteins from extremely small samples such as single cells. Further analysis such as mass spectroscopy could then serve to identify binding partners and modifications of the target proteins. | |
| "Being extendable to the detection of many other proteins, this method has the potential to be operated with extremely small sample volumes and can be incorporated into highly-integrated, autonomously operating molecular sorting devices" says Diez. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
