| Posted: Aug 09, 2006 | |
Nanoparticles stabilize proteins |
|
| (Nanowerk Spotlight) A new study presents a viable strategy to stabilize enzymes under conditions found in real world biocatalytic applications. This stabilization of proteins through gold nanoparticles occurs through two mechanisms: 1) binding of the protein in its active structure stabilizes that structure; 2) the gold particles lower the interfacial energy between air and water, thus diminishing the driving force for denaturation. The result is a functional biocatalyst that can be readily applied to biotechnological applications. | |
| Enzymes are highly specific and efficient biocatalysts with a wide range of biotechnological and industrial applications. The amphiphilic nature of proteins, however, leads to their instability at air–water and oil–water interfaces. Conditions that expose proteins to these interfacial environments, for instance through transport, printing, or mixing, can cause irreversible protein denaturation leading to a loss of activity. | |
| One way of increasing the stability of proteins is through immobilization of enzymes onto solid supports. Dr. Vincent Rotello and his group used nano-sized particles as scaffolds to support and stabilize the enzyme chymotrypsin. Rotello, the Charles A. Goessmann Professor of Chemistry at the University of Massachusetts, explained his group's recent work to Nanowerk: "The key finding is that we can stabilize proteins under conditions that would normally reduce or eliminate their activity." | |
| The research in Rotello's group is focused on increasing the understanding of the interface of biomolecules with materials. This research has applications in catalysis, as well as therapeutics, diagnostics, and useful materials. | |
| Like other enzymes, chymotrypsin has a natural tendancy to degrade at air–water surfaces, losing its structure and catalytic activity. According to Rotello, the enzyme can be stabilized by surrounding it with monolayer-protected gold nanoparticles. Therse are spherical nano-structures in which gold nanoparticles are surrounded by a negatively-charged shell of tetra(ethylene glycol) carboxylate ligands. | |
| "In previous studies" says Rotello, "we have demonstrated the electrostatic binding of α-chymotrypsin (ChT) to anionic nanoparticle surfaces featuring tetra(ethylene glycol) carboxylate ligands (Au-TCOOH). Significantly, the native structure of ChT is retained upon complexation with Au-TCOOH. More importantly, the enzyme–nanoparticle complex is still active towards neutral and cationic substrates, facilitating the application of these enzyme–nanoparticle conjugates as biocatalysts. | |
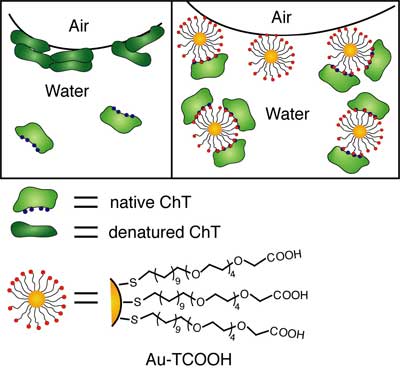 | |
| Schematic representation of ChT and ChT–AuTCOOH complex at air–water interface. (Source: Dr. Rotello, University of Massachusetts) | |
| In their current work ("Stabilization of α-chymotrypsin at air–water interface through surface binding to gold nanoparticle scaffolds") Rotello's group reports the stabilization of ChT at air–water interfaces through non-covalent binding to Au-TCOOH nanoparticles. | |
| Rotello’s work could have a significant impact in many areas such as biosensor development, where the stabilization of enzymes and proteins has been one of the key limiting factors in the field. | |
| "Enzymatic synthesis is a rapidly growing segment of the fine chemicals market" says Rotello. "Biocatalysis also provides a very "green" approach that could be applied to commodity chemicals." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
