| Posted: Oct 13, 2008 | |
The long road towards single molecule nanotechnology electronics |
|
| (Nanowerk Spotlight) Using single molecules as electronic components is the ultimate goal for future electronic nanotechnology devices (see for instance our Spotlight "Using quantum mechanics to turn molecules into transistors"). In order to explore the electronic properties of a single molecule, researchers have to make electrical contact between electrodes and molecules – and this has proven to be a big challenge. The problem is forming stable and reproducible molecular bridges and determining their electrical properties. It has already been shown that molecular bridges can be formed. However, their mechanical stability and reproducibility is usually low. | |
| Whether it is really possible to form a single stable molecular bridge, which can act as a 'normal' electrical component at room temperature has yet to be answered. An important step in this direction will be to identify all the mechanics involved in single atom contacts. | |
| A few years ago, scientists made important progress by contacting a single molecule using a scanning tunneling microscope (STM) in a liquid environment at room temperature ("Measurement of Single-Molecule Resistance by Repeated Formation of Molecular Junctions"). However, many details of the junction formation are still unknown. | |
| By using a similar method (mechanically controllable break junctions in stead of a scanning probe) a team of scientists in The Netherlands focused on an important detail of junction formation and tried to answer the question: what is the molecule's position just before a electrode-molecule-electrode bridge is formed? | |
| "We found evidence that this bridge is actually already being formed before it is observed, since its conductance is still being dominated by parallel metal bridges" Eek Huisman tells Nanowerk. "These details are very important for understanding and eventually improving successful formation of molecular junctions." | |
| Huisman, a researcher at the Zernike Institute for Advanced Materials at the University of Groningen, is first author of a recent paper in Nano Letters that discusses new aspects of molecular bridge formation in break junction experiments with gold molecules in solutions of dithiolated molecules ("Stabilizing Single Atom Contacts by Molecular Bridge Formation"). | |
| A combined research team from Huisman's Zernike Institute, led by Dr. Bart J. van Wees and the Kamerlingh Onnes Laboratory at Leiden University, led by Dr. Sense J. van der Molen, found that, in order to break a single atom contact, the electrodes have to be displaced 2-3 times longer in the presence of alkanedithiols as compared to the displacement when only solvent is present. | |
| In their experiments, the researchers focused on the moment right before the 'jump out of contact' – the instance where, as the STM tip is pulled carefully out of contact with the underlying gold substrate, the conductance suddenly jumps downward, indicating a break of the gold constriction. It is only then – when the metal bridge breaks – that metal-molecule-metal bridges become observable in the conductance. | |
 |
|
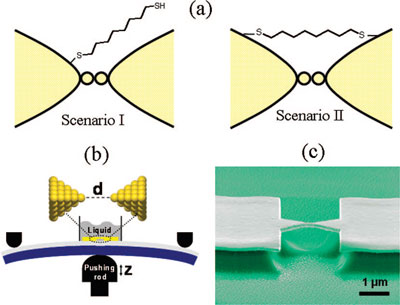
| (a) Two possibilities for the position of a dithiolated molecule just before breaking of the gold wire. In I, the molecule is attached to one side of the electrode only. In II, the molecule is attached to both electrodes. (b) Schematic drawing of the 'mechanically controllable break junctions' (MCBJ) technique showing the liquid cell on top of the microfabricated gold leads on the flexible substrate clamped in a three-point bending configuration. (c) Scanning electron micrograph showing the suspended gold bridge on top of the polyimide layer. (Reprinted with permission from American Chemical Society) | |
| Huisman says that this observation provides evidence for a scenario (Scenario II in above figure) in which a few molecules already span the atomically thin gold neck before breaking, thereby reinforcing the atomic contact. An alternative scenario (Scenario I in above figure) would have been that the metal-molecule-metal bridge is formed only after the jump out of contact, when the loose end of the molecule binds to the other electrode. | |
| This research is another typical example of the enormous challenges it will take to get to true atomic engineering. From where we are now, it looks like scientists will be busy for years to come to first develop the sound theoretical understanding of material properties at the single atom scale before anyone can seriously talk about nanoelectronics replacing established technologies like CMOS. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
