| Posted: Aug 30, 2006 | |
The flip side of using carbon nanotubes for environmental pollutants removal |
|
| (Nanowerk Spotlight) Carbon nanomaterials have been studied as superior sorbents for their potential environmental applications to remove pollutants such as organic pollutants, metals, fluorides and radionuclides. Most of these studies focused on the adsorption process and few dealt with the interfacial interactions of organic contaminants with carbon nanomaterials in aqueous media. However, understanding their desorption behavior as well is critical to evaluating environmental and health impacts of carbon nanomaterials. New research looks at the high adsorption capacity and reversible adsorption of PAHs (polycyclic aromatic hydrocarbons), many of which are suspected carcinogens, on CNTs. The findings imply the potential release of PAHs if PAH-adsorbed CNTs are inhaled by animals and humans, leading to a high environmental and public health risk. | |
| It was suggested that the toxicity of carbon nanomaterials is not only from their own potentially harmful nature but also from the toxic substances sorbed by them. Therefore, knowledge of toxic compound adsorption by carbon nanomaterials is critical and useful for risk assessment of these nanomaterials. This knowledge is also important for understanding the effect of the nanomaterials on the fate and transport of toxic pollutants in the environment, similar to other carbon materials such as soot and charcoal. | |
| Adsorption - desorption hysteresis is widely observed for the sorption of organic compounds by soils, sediments and charcoals. Such compounds include pesticides, chlorinated benzenes, and PAHs. Two types of hysteresis are often observed: reversible and irreversible hysteresis. | |
| So far, the desorption of organic contaminants by carbon nanomaterials, addressed only by limited research, shows significant desorption hysteresis of naphthalene and 1,2-dichlorobenzene from fullerene (C60). However, the mechanisms of desorption hysteresis for fullerene are still unclear. Furthermore, desorption from carbon nanotubes has not been investigated as yet. | |
| Dr. Baoshan Xing, professor in the Department of Plant, Soil & Insect Sciences at the University of Massachusetts, explained his recent research to Nanowerk: "The objectives of our study were (i) to test whether hysteresis occurs for PAHs desorption from carbon nanotubes in aqueous solutions, (ii) to compare the desorption similarities or differences between fullerene and carbon nanotubes because they have distinct geometries, and (iii) to examine the mechanisms of desorption hysteresis. Therefore, desorption of naphthalene, phenanthrene and pyrene by six carbon nanomaterials were investigated comparably. Fullerene, single-walled (SWCNT) and multi-walled carbon nanotubes (MWCNTs) were chosen because of their definite nanostructures, different size and surface areas, and diverse physical and chemical properties. PAHs were selected because of their notable concentrations in the environment, toxicity, and persistence." | |
 |
|
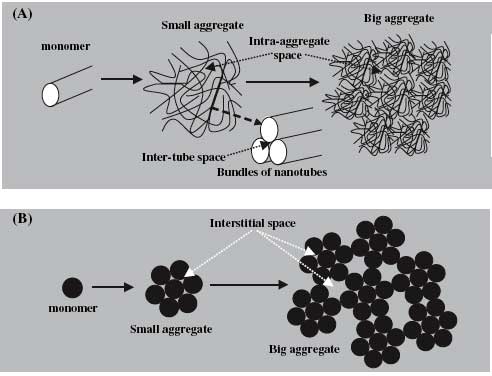
| Schematic aggregation process of fullerene and carbon nanotubes: (A) carbon nanotubes and (B) fullerene. Interstitial spaces of carbon nanotubes include intra-aggregate space and inter-tube space (A). Aggregation of spherical fullerene results in closed interstitial spaces in small aggregates and between small aggregates (B), while cylindrical carbon nanotubes cannot form closed interstitial spaces in their aggregates due to their length (A). (Reprinted with permission from Elsevier) | |
| As a result, Xing and his colleague Dr. Kun Yang observed irreversible PAH hysteresis for fullerene, but did not find any significant desorption hysteresis for carbon nanotubes. This difference was attributed to the materials' distinct geometries. For instance, some sorbed molecules will be blocked and entrapped in closed interstitial spaces between small fullerene aggregates, while for CNTs no closed interstitial spaces were formed in their aggregates due to their length, and no sorbed molecules are trapped. For both fullerenes and CNTs, the PAH adsorption on the external surface was reversible. The findings were published in a paper titled "Desorption of polycyclic aromatic hydrocarbons from carbon nanomaterials in water" (Environmental Pollution online on June 13, 2006). | |
| "High adsorption capacity and complete reversible adsorption of PAHs by CNTs imply the potential release of PAHs if PAH-adsorbed CNTs are inhaled by animals and humans, thus presenting high risk to the public health and environment" Xing concludes. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
