| Posted: Sep 05, 2006 | |
Nanotechnology in cosmetics - 2000 years ago...? |
|
| (Nanowerk Spotlight) These days we are debating if nanoparticles in sunblock and toothpaste are safe. The ancient Greeks and Romans didn't know about such things - but they already used nanotechnology in their cosmetics. A group of researchers in France showed that lead-based chemistry, which was initiated in Egypt more than 4000 years ago, could result in the synthesis of lead sulfide (PbS, galena) nanocrystals. With a diameter of about 5 nm, the appearance of these crystals is quite similar to PbS quantum dots synthesized by modern materials science techniques. | |
| Dr. Philippe Walter from the Centre de recherche et de restauration des musées de France (C2RMF-CNRS) in Paris explained these recent findings to Nanowerk: "For thousands of years, cosmetics have been used and were made by the judicious combination of naturally available minerals with oils, various creams, or water. Since the Greco-Roman period, organic hair dyes obtained from plants such as henna have been used, but other unusual formulas based on lead compounds, such as the recipes describing several methods to dye hair and wool black, were also common. It is remarkable that these Greco-Roman techniques have been used up to modern times." | |
| Walter, a Senior Research Scientist at the C2RMF-CNRS and head of the AGLAE and analytical chemistry goup, together with colleagues from the Laboratoire d'étude des microstructures from the French national aerospace research center and L'Oréal Research in France, as well as Argonne National Laboratory in the U.S., showed that an ancient dyeing process for blacking hair is a remarkable illustration of synthetic nanoscale biomineralization. | |
| "In contrast to modern nanotechnology, the dyeing process is characterized by basic chemistry methods and has been developed more than 2000 years ago with low-cost natural materials" Walter points out. | |
| The hair shaft contains three principal concentric histological regions: the cuticle, the cortex, and the medulla. The researchers found the presence of PbS particles in the cuticle and the cortex, which modifies the optical aspect of the hair shaft irreversibly. Nevertheless, the hair mechanical properties are essentially unaffected because of the extremely small size and volume fraction of the crystals. This opened the application of the dye formula for 2000 years. | |
| "What is particularly surprising in this reaction" says Walter, "is that, despite the structural complexity of hair and its relative chemical inertness, metal sulfide nanoparticles easily crystallize and get organized inside this biomaterial, i.e. in the hair shaft. It seems that sulfur-rich peptides in the matrix surrounding supramolecular- organized keratin proteins serve as nanoscale reactors." | |
 |
|
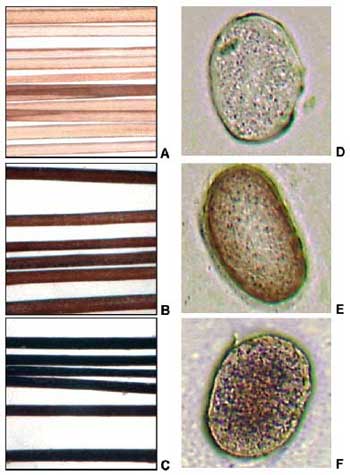
| Optical macrophotographs of hair (A-C) and microphotographs (D-F) of the corresponding transverse cross sections (thickness ) 10 ím), showing progressive blackening during treatment with lime and lead oxide in water (25 °C, pH ) 12.5). (A and D) nontreated, (B and E) 6h, (C and F) 72h. (reprinted with permission from American Chemical Society) | |
| For their experiments, which are described in a recent paper in Nano Letters ("Early Use of PbS Nanotechnology for an Ancient Hair Dyeing Formula"), the researchers used an ancient lead-based recipe to dye hair black. They found that the treated hair showed the presence of galena nanocrystals with an average diameter of under 5 nm. | |
| Natural black hair color is due to melanin clusters of ca. 300 nm dispersed within the colorless keratin-based cortex of hair. Lead-based hair-dying chemicals generate a kind of melanin substitute produced by synthesis of PbS inside hair; but these blackening PbS particles are much smaller than melanin clusters by 4-5 orders of magnitude in volume. | |
| With research like this, the scientists hope to better control the conditions for growth and organization of nanoparticles in organic matrix which ultimately could open new perspectives in the development of nanocomposites. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
