| Posted: Oct 31, 2008 | |
Nanotechnology is key to improving fuel cell performance |
|
| (Nanowerk Spotlight) Modern fuel cells have the potential to revolutionize transportation. Like battery-electric vehicles, fuel cell vehicles are propelled by electric motors. But while battery electric vehicles use electricity from an external source and store it in a battery, fuel cells onboard a vehicle are electrochemical devices that convert a fuel's chemical energy directly to electrical energy with high efficiency and without combustion (although the operation of a fuel cell vehicle is pollution free, the question is how the hydrogen for the fuel cell is produced – see Nanotechnology could clean up the hydrogen car's dirty little secret). | |
| One of the leading fuel cell technologies developed, in particular for transportation applications, is the proton exchange membrane (PEM) fuel cell, also known as polymer electrolyte membrane fuel cells – both resulting in the same acronym PEMFC. These fuel cells are powered by the electrochemical oxidation reaction of hydrogen and by the electroreduction of the oxygen contained in air. | |
| These fuel cells run at relatively low temperature (<100°C) and therefore need catalysts to generate useful currents at high potential, especially at the electrode where oxygen is reduced (the cathode of the fuel cell). Presently, platinum-based electrocatalysts are the most widely used in PEM fuel cell prototypes. However, this metal is expensive due to its limited supply and its price is highly volatile. This creates one of the major barriers preventing commercialization of PEMFCs – the lack of suitable materials to make them affordable. | |
| According to the U.S. Department of Energy (DOE), the system cost for automotive fuel cells has gone from $275 per kilowatt in 2002 to $95 per kilowatt in 2008 and is projected to be $60 per kilowatt in 2009. The target is $30 by 2015. The estimated cost for a gasoline engine is about $30 per kilowatt. | |
| Although nanotechnology promises cheap bipolar materials using nanocomposites, more efficient non-platinum electrocatalysts, and thermally stable and more durable membranes to become available in the near future, platinum still remains the workhorse of PEM fuel cells. This is why DOE has also established some performance targets for platinum use in PEM fuel cells for automotive application. These targets are 0.3 grams and 0.2 grams of platinum per kW of PEM fuel cell stack for 2010 and 2015, respectively. | |
| "In order to meet these targets, it is imperative to improve the specific activity of platinum-based fuel cell catalysts, especially at the cathode where the oxygen reduction reaction (ORR) is sluggish" Dr. Jean-Pol Dodelet tells Nanowerk. "This may be done by alloying platinum with one or more other metals. However, our work has shown that it is also possible to improve the specific activity of platinum by using platinum nanowires as electrocatalyst for ORR instead of the usual platinum nanoparticles." | |
| Dodelet is a professor at the INRS - Energy, Materials and Telecommunications at the Université du Québec in Canada. Together with his team – Drs. Shuhui Sun and Frederic Jaouen – they have demonstrated a simple room temperature aqueous phase synthesis of single-crystal nanowires of platinum on the nanospheres of a carbon black (a commonly used catalyst support in fuel cells). This use of carbon nanospheres as a substrate provides a cost-effective procedure for growing platinum nanowires. | |
| The resulting nanostructures – with the high-surface-area carbon black as the core and the electrocatalytically active platinum nanowires growing radially from the surface of the carbon particles – show enhanced catalytic activity for the ORR compared with a state-of-the-art platinum/carbon catalyst made of platinum nanoparticles. | |
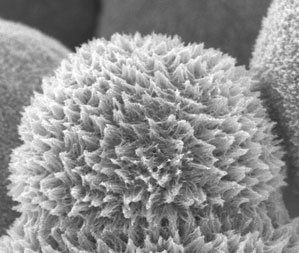 |  |
| A scanning electron microscopy (SEM) image of platinum nanostructures synthesized via a simple wet chemical method, at room temperature, using neither template nor surfactant. The nanostructures consisted of numerous single-crystal Pt nanowires with diameters of ca. 4 nm and lengths that may reach hundred nanometers. Right is detail view of left image. (Images: Dr. Dodelet/INRS) | |
| Although the team concedes that the growth mechanisms of nanowires and branched nanowires of platinum are not fully understood yet, they believe that anisotropic growth, preferentially in the (111) direction, is promoted by the very slow reduction rate at room temperature and the lowest energy principle. The length of the nanowires can be controlled by adjusting the reduction time of the platinum precursor, and their density on the carbon nanospheres by adjusting the weight ratio for platinum precursor to carbon. | |
| They report their findings in Advanced Materials ("Controlled Growth of Pt Nanowires on Carbon Nanospheres and Their Enhanced Performance as Electrocatalysts in PEM Fuel Cells"). | |
| "To the best of our knowledge, this is the first time that platinum nanowires have been used as electrocatalysts at the cathode of PEM fuel cells" says Dodelet. "It has been known for several years that the specific activity for oxygen reduction on platinum in acid medium is face-dependent, the most active faces being the low index single crystal surfaces: Pt(110), followed by Pt(111) and Pt(100). Platinum nanoparticles usually display all these low index single surfaces. As it was expected that platinum nanowires would display a smaller number of single crystal surfaces than platinum nanoparticles and would also have less structural defects than the particles, we undertook the synthesis of platinum nanowires according to a very facile route using the anisotropic growth of a platinum nanostructure performed in an environmental-friendly aqueous solution without the help of stabilizer or template." | |
| The platinum nanowires at the cathode of a membrane electrode assembly prepared in Dodelet's lab can reach much better performance in fuel cells than a commercial membrane electrode assembly. | |
| "We found that our in-house platinum nanowire catalyst shows a 50% higher mass activity than the commercial cathode" says Dodelet. "Quite surprisingly, this improvement occurred in spite of a 50% lower platinum area for the platinum nanowire catalyst. Taking into account both effects, a specific ORR activity of the platinum nanowire catalyst of 275 µA per square centimeter platinum (at 0.9 V) was calculated, which is threefold better than that of the commercial cathode when tested at the same fuel cell test station." | |
| The synthesis of these platinum nanowires is therefore an important step toward the fabrication of nanostructured platinum that would retain the high specific activity of bulk platinum. Consequently, Dodelet expects that platinum nanowires may favorably replace spherical platinum nanoparticles in any electrocatalytic application. | |
| He points out that some particular platinum alloy single crystal surfaces display higher specific activities than pure platinum in PEM fuel cells. "Therefore our next challenge is to obtain platinum alloy nanowires, especially those containing iron, copper, or nickel, in order to further improve the ORR activity of these catalysts in fuel cells." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
