| Posted: Sep 19, 2006 | |
Gaining a better understanding of fullerene growth mechanisms - one carbon molecule at a time |
|
| (Nanowerk Spotlight) With a better understanding of how fullerenes and nanotubes form, scientists and material engineers would be in a better position to provide conditions more favorable for the formation of a particular fullerene or a particular chirality and length nanotube. Researchers have used a number of computational and theoretical tools to explain the experimental observations and develop a picture of the dynamics for fullerene growth, yet no universally agreed model exists for the fullerene growth. To understand the phenomenon of fullerene growth during its synthesis, researchers modeled a minimum energy growth route using a semi-empirical quantum mechanics code. C2 addition leading to C60 was modeled and three main routes, i.e. cyclic ring growth, pentagon and fullerene road, were studied. | |
| Since the discovery of fullerenes by Kroto and Smalley in 1985, four basic fullerene growth mechanisms have been established: | |
| 1) graphene-to-fullerene transformation where sputtered or vaporized graphene sheet transforms into a closed-cage fullerene by ejecting a number of smaller carbon clusters; | |
| 2) the pentagon road or Limacon model, where an open-cage structure like corannulene grows by the addition of carbon atoms or molecules at its edges, and eventually the cage is closed; | |
| 3) ring-stacking, where carbon rings fuse together to form closed-cage fullerenes; and | |
| 4) the fullerene road, where a smaller closed-cage fullerene grows into a larger one by C1, C2 or C3 insertion into the cage. | |
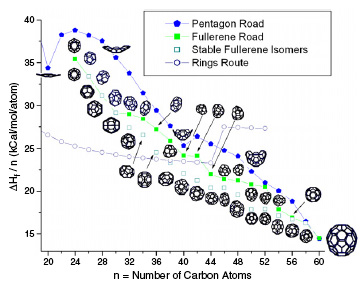 |
Growth of C60 from C24 is modeled by both pentagon and fullerene roads. Additionally, the growth of C60 ring is also presented. The ΔHf per carbon atom as a function of the number of carbon atoms is also presented. (Reprinted with permission from Institute of Physics Publishing) |
| Recent work supports the fullerene road as being more favorable than other mechanisms. As most of the fullerene formation mechanisms occur at high temperatures, molecular dynamics seems to be the best way to simulate fullerene growth. | |
| Sabih D. Khan from the Carbon-based Nanotechnology Laboratory in Islamabad, Pakistan, explained his group's recent simulations to Nanowerk: "C60 growth can be modeled by the sequential addition of C1, C2, C3 or larger carbon molecules, but experimental observations from emission spectra of regenerative sooting discharge indicate C2 to be an important constituent of carbonaceous plasma, which may lead to the formation of fullerenes. The scientific core of our finding is based on the addition of the C2s leading to the whole range of C clusters including the linear chains, rings, sheets and curved structures. We have also seen that open as well as closed structures can be formed by this route. The efficacy and applicability to closed cages has an edge over other C species' addition like C1s, C3s etc." | |
| The findings were described in a recent paper, titled "Modelling of C2 addition route to the formation of C60", appeared in the August 29, 2006 online edition of Nanotechnology. | |
| What Khan and his co-author Dr. Shoaib Ahmad found was that that the end product of C60 may be the result of competing structures that include rings, corannulene and fullerenes from C2-rich plasma by C2 addition. | |
| "The growth initially starts with linear carbon chains and, at n = 10, linear chains transform into rings, which further grow by C2 ingestion" says Ahmad. "At around n = 38, the rings appear to transform into fullerenes, and from there onwards the growth of C60 is achieved by the fullerene road." | |
| The researchers found that the ring-to-fullerene transition may be reached much earlier than n = 38 in a collision-dominated environment during fullerene synthesis. At n > 38, the fullerene road (close caged growth) is more favorable than the pentagon road (open caged growth) hence, if insufficient energy is available, fullerene formation is more favored than the cap formation, which may lead to the nanotube growth. | |
| These findings may help dealing with creating the right conditions for cage closure and for further control on the formation of a particular cage, C60 versus C70, for example. | |
| Ahmad points out that if one understands the formative stages and the routes to cage closure then one could control, alter and modify this behavior. | |
| "This may lead to a rich and powerful tool for the control of the physical properties of the C cages" he says. "This control, if achievable, may be extended to nanotubes and the desired chirality, tube diameter and length may be obtained." | |
| "A clear message from our work is that the pentagon road is not the energetically favored route for fullerene formation, while the single-walled nanotube growth based on the pentagon road needs to be re-investigated" Ahmad concludes. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
