| Posted: Dec 10, 2008 | |
Sun powered bio-hydrogen production moves closer |
|
| (Nanowerk Spotlight) The hydrogen that will power tomorrow's cars is not a naturally occurring resource that can be tapped by drilling a hole in the ground. Hydrogen has to be produced, and that can be done using a variety of resources. The cleanest by far of course would be renewable energy electrolysis: using electricity to split water into hydrogen and oxygen; this electricity could be generated using renewable energy technologies such as wind, solar, geo- and hydrothermal power. The dirtiest, at least until highly efficient carbon capture and sequestration technologies are developed, is the gasification of coal. As it stands, most of today's hydrogen production is 'dirty' – it is produced from methane in natural gas using high-temperature steam in what is called steam methane reforming. | |
| Many research groups around the world are working hard on developing cheap, clean and efficient technologies to produce hydrogen from water, particularly using sunlight (artificial photosynthesis). This would be the ultimate clean, renewable and abundant energy source. However, to become commercially viable, fuel cells have to overcome the barrier of high catalyst cost caused by the exclusive use of expensive platinum and platinum-based catalysts in the fuel-cell electrodes – the reason is that platinum is the most efficient electrocatalyst for accelerating chemical reactions in fuel cells. | |
| Apart from high cost, platinum has several other disadvantages (e.g. lack of selectivity; poisoning by trace inhibitors) and some scientists are focusing on a biological approach to hydrogen production. It was found that platinum catalysts can be replaced with hydrogenase enzymes that have nickel and iron in their active sites. | |
| Hydrogenases are biological catalysts that produce or oxidize hydrogen. They contain active sites with iron ([FeFe]), or nickel and iron ([NiFe]). They are produced in many microbial organisms, with slight differences from species to species. | |
| "Hydrogenases are often highly active but it is not so straightforward to use them for any technological application" Fraser Armstrong tells Nanowerk. "As enzymes, they are relatively unstable; in particular, [FeFe]-hydrogenases tend to be very oxygen sensitive. The [NiFe]-hydrogenases are less oxygen sensitive but are also less active in producing hydrogen, partly because they are strongly inhibited by hydrogen. We identified a hydrogenase with an unusual selenium-containing amino acid at the active site, which combines hydrogen production activity with a reasonable degree of tolerance to oxygen." | |
| As part of their ongoing enzyme-fuel cell and biohydrogen production projects, Armstrong's group at the University of Oxford in the UK are trying to understand principles of biohydrogen systems at the molecular level. They have previously described their system as a concept. Now, they have addressed this development in a practical context and demonstrated a rational photochemical hydrogen cell that produces hydrogen under visible light irradiation without resort to rigorous anaerobicity. | |
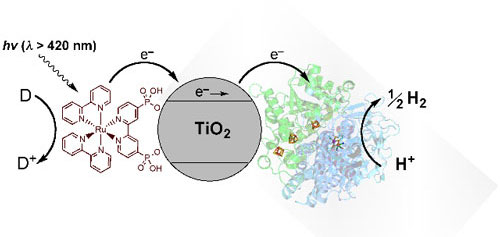 |
|
| Schematic representation of visible-light driven hydrogen evolution with Ru–TiO2-Hydrogenase, i.e., [NiFeSe]-hydrogenase attached to Ru dye sensitized titanium dioxide nanoparticles. Upon excitation by visible light in the presence of a sacrificial electron donor D, RuP injects an electron into the conduction band of TiO2. The electrons are transferred directly to the adsorbed [NiFeSe]-hydrogenase, thereby reducing H+ from the buffered aqueous solution. (Image: Erwin Reisner, University of Oxford) | |
| "The hydrogenase we identified was a good subject with which to assemble a system in which a hydrogenase attached to a semi-conducting nanoparticle was used to produce hydrogen when supplied with electrons from a photosensitizer – a dye that can be electronically excited by light – also attached to the particle," explains Erwin Reisner, a postdoc in Armstrong's group and first author of the team's report on their findings ("Catalytic electrochemistry of a [NiFeSe]-hydrogenase on TiO2 and demonstration of its suitability for visible-light driven H2 production"). Working with Armstrong and Reisner was Juan C. Fontecilla-Camps from the Laboratoire de Cristallographie et Cristallogenèse des Protéines at the Université Joseph Fourier, Grenoble, France. | |
| Armstrong says that his team's recent work is a first stage to a full demonstration of a rational enzyme-based particulate water-splitting system for photosynthesizing hydrogen. | |
| As a test enzyme for their light-powered hydrogen production system, the researchers used [NiFeSe]-hydrogenase from the bacterium Desulfomicrobium baculatum. Reisner explains that this enzyme is significant for several reasons: "First, it is an efficient hydrogen producer that is not as strongly inhibited by hydrogen as are the more common [NiFe]-hydrogenases. Second, compared to conventional [NiFe]-hydrogenases so far studied, it converts only very slowly (under oxidizing, anaerobic conditions) to an inactive oxidized state. Third, and very significantly, it can even sustain electrochemical hydrogen production in the presence of at least 1% oxygen, a property that is highly desirable for practical purposes and essential in any future developments where water is used as electron donor, i.e. true water splitting. Fourth, it binds strongly and persistently, in a fully electroactive state, to titanium dioxide." | |
| While similar systems have been described before, this experiment by the University of Oxford scientists is superior because the enzyme is an oxygen-tolerant hydrogen producer and visible (sun)light is used instead of ultra-violet light. | |
| "The useful properties of the [NiFeSe]-hydrogenase we used, in particular its stability on titanium dioxide and some oxygen tolerance for hydrogen production, should allow us to extend our work to include a water oxidation catalyst for biological water splitting," says Armstrong. | |
| Apart from the need for a cheap catalyst for water oxidation, stability of the system, and practical challenges of scaling up are some of the main hurdles that still need to be overcome before we can expect robust hydrogenase–nanoparticle devices, including development of mesoporous 3D electrodes for enzyme-fuel cells or biosensors. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
