| Posted: Jan 20, 2009 | |
Governing the risk of nanotechnology in food and cosmetics |
|
| (Nanowerk Spotlight) In case you want to get up to date on what's happening around the world with regard to the development of risk governance for nanotechnology applications in food and cosmetics, a new report just out from the International Risk Governance Council (IRGC) provides a good overview. An early version of this report was originally written as a briefing paper for an expert workshop organized by the IRGC in 2008. It is also a companion to the IRGC Policy Brief due for publication in early 2009. While this report does not include any primary research, is is a useful primer for anyone who wants to get an overview of what is happening in this area. | |
| IRGC is an independent organization whose purpose is to help the understanding and management of global risks that impact on human health and safety, the environment, the economy and society at large. The organization's focus on risk governance strategies for nanotechnology applications in food and cosmetics is based on rising public concerns: | |
| "Qualitative surveys of consumer opinion provide evidence of a positive to indifferent attitude towards nanotechnologies and their application, with one exception : foods. Concerns about cosmetics are also rising and consumer advocacy groups and independent experts have recommended that more risk assessments should be conducted before cosmetics containing nanoscale materials are put on the market. Public authorities in several countries have stressed the need for extended risk assessments and careful oversight." | |
| Consequently, the IRGC's nanotechnology project has the following objectives | |
|
|
|
| In the absence of reliable data, the IRGC author group (Dr. Antje Grobe, Professor Ortwin Renn and Alexander Jaeger of Dialogik) has used a Nanowerk chart from our Spotlights "Nanotechnology food coming to a fridge near you " and "The promises of food nanotechnology" where we provide an overview of what nanotechnology applications are currently being researched, tested and in some cases already applied in food technology. | |
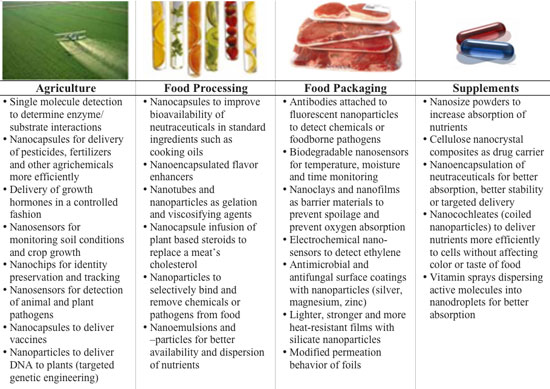 |
|
| Examples for nanotechnology applications in food and agriculture (Source: Nanowerk) | |
| After describing the use of nanomaterials in food and cosmetics, summarizing opinion research on public perception and reviewing the regulatory background and legal requirements for risk assessment, the report highlights risk assessment studies for three sample nanoscale materials: synthetic amorphous silica, titanium dioxide, and encapsulated vitamins. | |
| The authors then review the currently available codes and frameworks that provide guidelines for risk assessment, management and communication: | |
|
|
|
| The report's authors suggest that voluntary codes such as these offer an alternative to regulation (although we have outlined some of the problems with this approach in a previous Spotlight: "Implementing successful voluntary nanotechnology environmental programs appears to be a challenge"). The main reason for that is that regulation is extremely difficult to design because of the problems of defining novel nanoscale materials. | |
| "Although new regulations specific to nanotechnology, whether in food and cosmetics or in other sectors such as medicine, appear unlikely at the present time, industry would be well advised to establish an enforceable, transparent and inclusive process of self-regulation through a voluntary code. However, this step may not satisfy concerned NGOs: 'Voluntary initiatives are wholly inadequate to oversee nanotechnology... the public overwhelmingly prefers mandatory governmental oversight to voluntary initiatives'." | |
| The report "Risk Governance of Nanotechnology Applications in Food and Cosmetics" (pdf, 1.4 MB) can be downloaded from the IRGC's website. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
