| Posted: Oct 19, 2006 | |
New potent nanoassemblies to fight cancer and HIV |
|
| (Nanowerk Spotlight) Nucleoside analogues, which are a class of therapeutic agents, display significant anticancer or antiviral activity by interfering with DNA synthesis. They work by incorporating into the elongating DNA strands and terminating the extension process. However, they also affect normal cell growth, such as bone marrow cells, so there can be significant toxic effects. Further limitations to their use are relatively poor intracellular diffusion, rapid metabolism, poor absorption after oral use, and the induction of resistance. French and Italian researchers have now come up with a completely new approach to render anticancer and antiviral nucleoside analogues significantly more potent. By linking the nucleoside analogues to squalene, a biochemical precursor to the whole family of steroids, the researchers observed the self-organization of amphiphilic molecules in water. These nanoassemblies exhibited superior anticancer activity in vitro in human cancer cells. | |
| Professor Patrick Couvreur, director of the Physico-chimie, Pharmacotechnie et Biopharmacie department at the Université de Paris-Sud, and a team of researchers from the Università degli Studi di Torino/Italy and the Laboratoire de Neurovirologie in Fontenay-aux-Roses/France, have discovered that the linkage of nucleoside analogues to squalenic acid ("squalenization"), the acyclic isoprenoid chain of squalene, leads to squalenic amphiphilic prodrugs which self-organize in water as nanoassemblies of 100-300 nm, irrespective of the nucleoside analogue used and the location of covalent linkage. Based on these findings they conceived a new strategy to increase the therapeutic index of the nucleoside analogues and to administer them more efficiently. | |
| Couvreur explained the findings to Nanowerk: "Development of this new platform has been first demonstrated with gemcitabine, a major anticancer drug with broad spectrum activity. The nanoassemblies formed by the squalenization of gemcitabine were found about 6 to 8 times more cytotoxic than gemcitabine itself in vitro on two human cancer cell lines and they exhibited in vivo in aggressive leukemia a dramatically higher anticancer effect than gemcitabine (both after intravenous and oral administration)." | |
 |
|
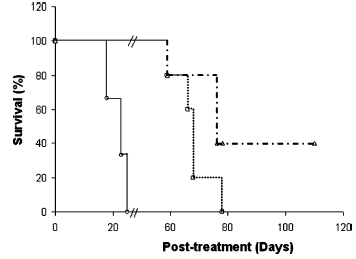
| Survival of F344 Fischer rats bearing RNK-16 LGL leukemia after oral treatment with 15 mg kg-1 gemcitabine and its equivalent as SQdFdC NA. Untreated (solid line), gemcitabine (••••), SQdFdC (squalenoyl gemcitabine) nanoassembly (–•–•–•). n=5. The Kaplan-Meier test was significant for RNK-16 LGL leukemia bearing rats (P<0.05). (Reprinted with permission from the American Chemical Society) | |
| "We extended the concept of "squalenization" to other nucleoside analogues with antiretroviral activity like ddC and ddI" says Couvreur. "All these squalenated molecules self-organized in water into nanoassemblies of 100-300 nm and were found at least two-times more potent than the corresponding parent molecules to inhibit the virus spread in primary cultures of HIV-1-LAI infected lymphocytes." | |
| Thus, the squalenization of nucleoside analogues is an original platform for the discovery of more potent anticancer and antiviral nanomedicines able also to fight against resistances. This is a real breakthrough since there is nothing similar reported before. | |
| Couvreur is very excited about the prospects for squalenization as a new technology platform: "It is a fascinating discovery with high scientific significance which could lead to the discovery of new drugs to cure diseases as severe as cancer and AIDS". | |
| Based on this concept of squalenization of nucleoside analogues, Couvreur is setting up a start-up company in France, called MEDSQUAL. | |
| The researchers reported their novel process in a paper, titled "Squalenoyl Nanomedicines as Potential Therapeutics", that was published in the October 7, 2006 online edition of Nano Letters. | |
| A previous Nanowerk Spotlight ("Nanocarriers could become an alternative to brain surgery") about Dr. Couvreur's work dealt with a novel targeted nanoparticulate drug delivery system for the brain. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
