| Posted: Mar 03, 2009 | |
Observing all the intermediate steps of a chemical reaction |
|
| (Nanowerk Spotlight) Over the past 25 years, Scanning Tunneling Microscopy (STM) has brought us extremely detailed images of matter at the molecular and atomic level. STM, which is a non-optical technique, works by scanning an electrical probe over a surface to be imaged to detect a weak electric current flowing between the tip and the surface. The STM allows scientists to visualize regions of high electron density and hence infer the position of individual atoms and molecules on the surface of a lattice. | |
| Researchers also believe that the strength of time-lapsed high-resolution STM work to unravel complex surface reactions would allow them to achieve one of the 'Holy Grails' within the area of surface science, which is to directly observe chemical reactions at the atomic scale. A research team in Denmark has now shown that, by means of high-resolution STM studies in conjunction with density functional theory calculations (DFT), it is possible to follow the intermediate steps of a complex oxidation reaction. | |
| "By combining time-lapsed, high-resolution STM studies and DFT calculations we have revealed unprecedented details about the chemical reaction of oxygen with hydrogen adatoms on rutile titanium dioxide," Stefan Wendt tells Nanowerk. | |
 |
|
| STM movie (Source: Stefan Wendt, iNANO) | |
| Wendt, a post-doc at the Interdisciplinary Nanoscience Center (iNANO) at the University of Aarhus in Denmark, stresses that it is not easy to get such high-resolved STM movies but that there is a great potential to unravel how a surface catalyzed reaction occurs on an oxide surface. | |
| "Using averaging techniques such as vibration spectroscopy, which can be applied for the study of powder samples, it is often impossible to identify intermediate species on well-characterized single crystal surfaces," says Wendt. "In our paper (Observation of All the Intermediate Steps of a Chemical Reaction on an Oxide Surface by Scanning Tunneling Microscopy), we demonstrate, for the first, time how time-lapsed STM imaging can successfully reveal unprecedented details about the intermediate steps for a surface catalyzed reaction." | |
| The paper was first-authored by Jesper Matthiesen who is now at Pacific Northwest National Laboratory. The team, which included members of iNANO's Scanning Probe Microscopy Group, led by Flemming Besenbacher, and Theoretical Surface Science Group, led by Bjørk Hammer, specifically studied the oxidation of hydrogen adatoms by oxygen molecules on rutile titanium dioxide (110) by high-resolution STM. | |
| Wendt explains that, by using time-lapsed STM imaging, they have unraveled the individual reaction intermediates of HO2, H2O2, and H3O2 stoichiometry and the final product – pairs of water molecules, [H2O]2. | |
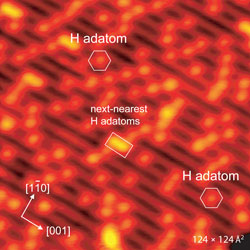 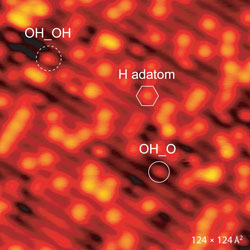 |
|
| STM images of the h-TiO2(110) surface before (left) and after 4 L oxygen exposure at ∼165 K (right). Symbols in image on the left indicate single H adatoms (hexagon) and next-nearest H adatoms (rectangle, full line). The circles in he image on the right indicate the newly formed species in the titanium troughs. (Image: Stefan Wendt, iNANO) | |
| "Because of their different appearance and mobility, these four species were clearly discernable in an STM movie" he says. "The interpretation of the STM results is corroborated by density functional theory calculations (DFT). Our results demonstrate that it is possible to reach a long-standing goal for surface scientists, namely to directly 'watch' chemical reactions at the atomic scale." | |
| Until now, scientists have used STM to induce reactions on surfaces and, e.g., to follow reaction fronts of catalytic reactions. However, direct visualization of several consecutive reaction steps at the atomic scale on an oxide surface has not been achieved so far. | |
| Another accomplishment of the iNANO team concerns the type of chemical reaction that they observed – the oxidation of hydrogen adatoms on a very important material, titania. | |
| Wendt points out that the complex reaction unraveled in their study – consisting of several transfers of hydrogen atoms to adsorbed oxygen on the titania surface, leading to the formation of water – is of great interest for applications in the fields of catalysis and photo-catalysis. | |
| "So far, the mechanisms of this reaction was unknown or, at best, speculative" he says. "Furthermore, our work unravels the role of traces amounts of water in the reaction, which is of general relevance, since water is always present at realistic reaction conditions. Previously, the important role of water has been noted in more applied catalysis studies, but was not understood." | |
| Based on spectroscopic techniques, it was known before that water is the reaction product when oxygen molecules react with hydrogen atoms on titanium dioxide surfaces. However, the previously proposed reaction mechanism was speculative and simplified. | |
| The iNANO scientists identified a reaction mechanism that differs from the one proposed previously. Wendt explains that the difference with respect to the mechanism traces back to the role of co-adsorbed water to facilitate the diffusion of hydrogen adatoms, which was not known at that time. | |
| The oxygen/hydrogen reaction addressed by Wendt and his collaborators is very crucial for numerous reactions on titania supported metal clusters and photo-catalytic reactions on titania nanoclusters. Both oxidation and reduction reactions on titania-based catalysts are very important. Wendt notes that, for example, reduction reactions are crucial for renewable energies for the future – i.e. hydrogen production – and oxidation reactions are important for cracking of organic waste, i.e. for clean-up processes. Therefore, there is great interest to understand reactions with oxygen and hydrogen and water and its intermediates present on the surface. | |
| Research work like this one is motivated by the promising properties of titania in many fields such as photo-catalysis, heterogeneous catalysis, solar cells, self-cleaning glasses, biocompatible materials and electronic devices. In addition, the particular titania face (110) that the team studied has become a prototypical surface for transition metal oxides. Thus, the hope is that the knowledge gained about this specific oxide surface will help also to better understand reactions on other important oxide surfaces. | |
| More realistic models for reactions occurring on oxide catalysts and oxide supported metal catalysts may prove extremely useful in optimizing the reaction conditions and the production of more efficient catalysts and photo-catalysts. | |
| According to Wendt, a possible future direction of their research is to study how UV light illumination influences the oxygen/hydrogen reaction on titania surfaces. "Also, although challenging, the influence of co-adsorbed metal clusters (gold for instance) would be very interesting in this regard," he says. "Recall that a long-standing goal is actually to split water into oxygen and water, rather than to produce water from oxygen and water. Thus, we need to study how we can reach that the reaction runs in the opposite way as it is doing naturally on reduced titania materials. The latter should be possible by the use of UV light and well-designed titania-based materials." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
