| Posted: Jul 21, 2014 |
The stability of gold clusters: Every ligand counts
|
|
(Nanowerk News) By colliding ultra-small gold particles with a surface and analyzing the resulting fragments, a trio of scientists at Pacific Northwest National Laboratory discovered how and why the particles break. This information is important for controlling the synthesis of these tiny building blocks that are of interest to catalysis, energy conversion and storage, and chemical sensing. The team showed that the particles break along three competing pathways. The path chosen by a specific cluster depends on the amount of energy that binds it together as well as whether a given pathway leads to rapid or slow fragmentation. Particles with eight gold atoms wrapped with six ligands or phosphorus-based strands proved exceptionally stable in part because the ligands tightly bind to the gold core.
|
|
“There is substantial interest in many disciplines that wish to control the synthesis of metal clusters,” said Dr. Julia Laskin, a physical chemist and Laboratory Fellow who led the study. “These particles’ optical and electronic properties make them promising candidates for applications in catalysis, photovoltaics, and drug delivery.”
|
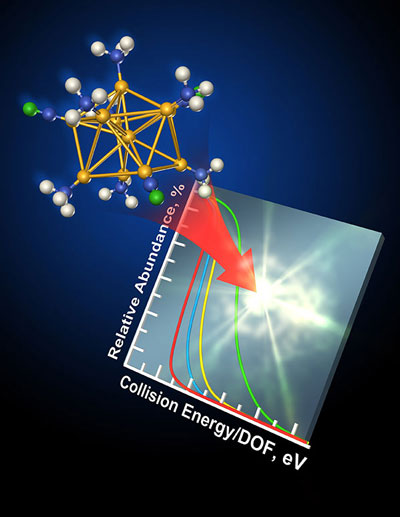 |
| What determines the stability of gold clusters, which may be the building blocks or new energy technologies? By colliding the ultra-small particles with a surface and analyzing the resulting fragments, scientists found that the binding energy between the gold center and the surrounding molecular framework is key. This work was done by a team at PNNL, using resources at DOE’s EMSL, a national scientific user facility.
|
|
Why It Matters
|
|
Today’s materials will not likely solve our nation’s mounting energy challenges. They simply don’t have the necessary properties to produce more efficient catalysts or solar cells that create, store, and use energy on a massive scale. Employing ultra-small particles, scientists are synthesizing highly tailored materials with the desired stability and chemical reactivity. However, in many cases, researchers lack basic thermodynamic and kinetic information about how the particles behave. The study done at PNNL -- which is the first of its kind -- provides fundamental knowledge that allows researchers to control the synthesis of gold particles or other nanosized clusters in a more rational manner.
|
|
"By understanding the fundamentals of cluster synthesis through thermochemistry, we may control and exploit these processes to create superior materials for a variety of energy-related applications," said Dr. Grant Johnson, a PNNL physical chemist who worked on the study ("Size-dependent stability toward dissociation and ligand binding energies of phosphine ligated gold cluster ions").
|
|
Methods
|
|
The team began by synthesizing a variety of ionic gold clusters in solution. The clusters had 7, 8 or 9 gold atoms and 6 or 7 triphenylphosphine ligands. Their chemical formulas were Au7L62+, Au8L62+, Au8L72+ and Au9L72+ (Au = gold, L = ligand). They filtered out the clusters of interest one by one in the gas phase using mass spectrometry. “We isolate clusters of the exact size that we want,” said Johnson. “In solution, this is very challenging to accomplish -- so measurements are typically made on poorly defined distributions of particles.”
|
|
Next, they collided the clusters they selected with a special surface inside the mass spectrometer in a process known as surface-induced dissociation. The instrument showed the fragments produced when the clusters fragmented at different collision energies and times. Using a unique modeling approach developed by Laskin, they determined the energy involved in breaking the clusters apart and whether each route to fragmentation is rapid or slow for a specific cluster.
|
|
They found the clusters dissociate through 1 of 3 competing pathways. The particles may break
|
|
With the loss of an uncharged phosphine ligand.
Through asymmetrical fission, with a gold atom and two ligands breaking off.
Through more symmetrical fission, with two fragments having nearly the same number of gold atoms and ligands.
|
|
The team showed that a cluster containing 8 gold atoms and 6 ligands is exceptionally stable compared to other clusters. It turns out that this cluster is also the predominant species formed during the early stages of reduction synthesis in solution. This observation demonstrates that experiments with gas-phase ions are indispensable for understanding the thermochemistry of complex processes occurring in solution.
|
|
The study also provides theorists with benchmark values for their calculations and simulations of ligated gold clusters. Experimental benchmarks give theorists important complementary information that aids them in selecting the most computationally efficient and accurate methods.
|
|
What’s Next
|
|
The team expects that the surface-induced dissociation experiments will play an increasing role in understanding the initial steps of cluster nucleation and growth in solution. They are currently examining different ligated clusters to understand how the spatial arrangement of the atoms in the ligands alters the abundance of clusters formed in solution and their stability in the gas phase.
|

