| Posted: Jul 28, 2014 |
Nanoparticle research - simulating the Invisible (w/video)
|
|
(Nanowerk News) Panagiotis Grammatikopoulos in the OIST Nanoparticles by Design Unit simulates the interactions of particles that are too small to see, and too complicated to visualize. In order to study the particles’ behavior, he uses a technique called molecular dynamics. This means that every trillionth of a second, he calculates the location of each individual atom in the particle based on where it is and which forces apply. He uses a computer program to make the calculations, and then animates the motion of the atoms using visualization software. The resulting animation illuminates what happens, atom-by-atom, when two nanoparticles collide.
|
|
Grammatikopoulos calls this a virtual experiment. He knows what the atoms in his starting nanoparticles look like. He knows their motion follows the laws of Newtonian physics. His colleagues have seen what the resulting particles look like after collision experiments. Once his simulation is complete, Grammatikopoulos compares his end products with his colleagues to check his accuracy.
|
|
Grammatikopoulos most recently simulated how palladium nanoparticles interact, published in Scientific Reports on July 22, 2014 ("Coalescence-induced crystallisation wave in Pd nanoparticles").
|
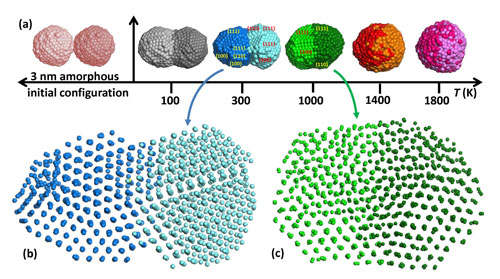 |
| Grammatikopoulos simulated two palladium nanoparticles colliding at different temperatures. The hotter the temperature, the more homogenous the resulting product, and the further the atoms in the particle crystallize. (click on image to enlarge)
|
|
Palladium is an expensive but highly efficient catalyst that lowers the energy required to start many chemical reactions. Researchers can make palladium even more efficient by designing palladium nanoparticles, which use the same mass of palladium in tinier pieces, increasing surface area. The more surface area a catalyst has, the more effective it is, because there are more active sites where elements can meet and reactions can occur.
|
|
However, shrinking a material to only a few nanometers can change some of the properties of that material. For example, all nanoparticles melt at cooler temperatures than they would normally, which changes what happens when two particles collide. Ordinarily, two particles will collide and release a small amount of heat, but the particles remain more or less the same. But when two nanoparticles collide, sometimes the heat released melts the surface of the two particles, and they fuse together.
|
|
|
|
Palladium Crystallization at 300K. Grammatikopoulos created this simulation of palladium nanoparticles colliding at 300 Kevin, or about 27 degrees Celsius. The nanoparticles meet, then fuse, then crystallize in orderly planes.
|
|
Grammatikopoulos simulated palladium nanoparticles colliding and fusing at different temperatures. He determined that each time the particles fused, their atoms would start to crystallize into orderly rows and planes. At higher temperatures, the particles fuse into one homogeneous structure. At lower temperatures, the products look like classic snowmen, with a few parts that had crystallized with different orientations.
|
|
“The simulation gives you an understanding of physical processes,” said Grammatikopoulos. Before his research, Grammatikopoulos could not explain why all the palladium nanoparticles his lab created had a crystalline structure. Furthermore, he noticed that many palladium nanoparticles grew protrusions, giving the particles a lumpy shape. “Since the protrusions stick out, they bond more easily with other molecules,” Grammatikopoulos explained. “I’m not sure yet if it’s beneficial, but it’s definitely affecting the catalytic properties.”
|
|
|
|
Palladium Crystallization at 1000K. Grammatikopoulos created this simulation of palladium nanoparticles colliding at 1000 Kevin, or about 727 degrees Celsius. At this hot temperature, the nanoparticles meet, fuse, and crystallize, forming one large homogenous product.
|
|
This study establishes some ground rules and explains certain properties of palladium nanoparticles. Understanding these properties could help design other nanoparticles out of other materials that would rival palladium’s abilities as a catalyst. Palladium plays a role in thousands of important reactions, from making drugs to creating new biofuels. For example, Prof. Mukhles Sowwan’s Nanoparticles by Design Unit and Prof. Igor Goryanin’s Biological Systems Unit at OIST are working with palladium-catalyzed reactions to improve the efficiency of microbial fuel cells. Better palladium nanoparticles will propel this research forward.
|
|
“We need to understand the basic science,” explained Sowwan, who is Grammatikopoulos’ advisor. Sowwan says that the field of nanoscience is only starting to move towards applying the research, because there is still so much to learn about the properties of nanoparticles. “If you build something without understanding the basics,” Sowwan said, “you will not be able to explain the results.”
|

