| Posted: Aug 25, 2014 |
A newly discovered gold nanocluster promises rich chemical yields
|
|
(Nanowerk News) Old thinking was that gold, while good for jewelry, was not of much use for chemists because it is relatively nonreactive. That changed a decade ago when scientists hit a rich vein of discoveries revealing that this noble metal, when structured into nanometer-sized particles, can speed up chemical reactions important in mitigating environmental pollutants and producing hard-to-make specialty chemicals. Catalytic gold nanoparticles have since spurred hundreds of scientific journal articles. With the world catalyst market poised to hit $19.5 billion by 2016, gold nanoparticles may find commercial as well as intellectual importance, as they could ultimately lead to novel catalysts for energy, pharmacology and diverse consumer products.
|
|
But before gold nanoparticles can be useful to consumers, researchers have to make them both stable and active. Recently, scientists learned to make tiny, highly ordered clusters with very specific numbers of gold atoms that are stabilized by compounds called ligands. These stabilized gold clusters plus ligands may be thought of as large molecules. In collaboration with scientists from Carnegie Mellon University, researchers at the Department of Energy’s Oak Ridge National Laboratory have found one new gold molecule, a catalyst containing exactly 25 gold atoms, that is powerful as well as sophisticated. It catalyzes the conversion of a variety of molecules, including the transformation of poisonous carbon monoxide into harmless carbon dioxide, a reaction that may find application in devices near gas flues or wood-burning stoves. Unfortunately, the ligands that create and stabilize the engineered clusters also block the very sites needed to catalyze the conversion of carbon monoxide into carbon dioxide.
|
|
“The ligands are double-edged swords,” said study leader Zili Wu of ORNL, whose investigation was conducted in ORNL’s catalysis group, which is led by Steve Overbury. “We’re interested in using gold clusters as catalysts or catalyst precursors. Ligands on the one hand stabilize the gold particle structure but on the other hand decrease their catalytic performance. Balancing those two factors is the key to creating a new catalytic system. One way is to utilize a metal oxide (here, cerium oxide) as an inorganic ligand to stabilize the gold clusters when the organic ligand has to be removed for catalysis.”
|
|
Many catalytic systems consist of metal particles with catalytic properties placed on a metal oxide support with catalytic properties of its own. The metal and metal oxide work together to create a new type of catalytic activity. “We’re trying to understand how that happens,” Wu said.
|
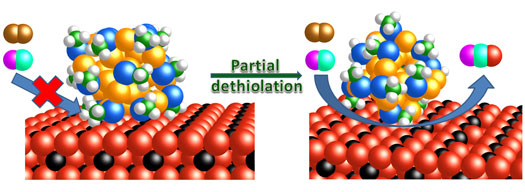 |
| The reaction mechanism of carbon monoxide oxidation is shown over intact and partially ligand-removed gold nanoclusters supported on cerium oxide rods. (Image: © ACS)
|
|
Their study, published in the Journal of the American Chemical Society ("Thiolate Ligands as a Double-Edged Sword for CO Oxidation on CeO2 Supported Au25(SCH2CH2Ph)18 Nanoclusters"), described how ligands enabled the gold nanocluster to dock on a cerium oxide support shaped like a rod. The catalysts produced were all identical. The researchers would like to engineer future oxide supports in the shapes of cubes or octahedra to find out how those nanostructures could alter the configuration of the gold and the reactivity of the final component system. Better understanding of stabilizing agents may aid design of novel catalysts for critical chemical reactions including oxidation, hydrogenation and coupling.
|
|
Carnegie Mellon Professor Rongchao Jin, his student Chenjie Zeng and ORNL postdoctoral fellows Amanda Mann and Zhen-An Qiao synthesized the gold clusters. Mann made the cerium oxide rods. Wu and Mann placed the gold clusters on the supports and performed chemical reaction studies. David Mullins of ORNL performed measurements of extended X-ray absorption fine structure to learn how sizes of clusters change with temperature. ORNL’s Larry Allard verified the nature of the structures with aberration-corrected microscopy, and De-en Jiang, formerly of ORNL but now at the University of California–Riverside, used the Oak Ridge Institutional Cluster to computationally explore structures of ligand-bound gold clusters.
|
|
Activating gold
|
|
“These ligands affect the reactivity—they essentially poison the gold surface—so the gold really has to be activated,” Overbury, the study’s senior author, explained. “We put the gold onto a support, and it’s got these ligands protecting it. We have to remove those ligands, so we basically heat this [gold nanocluster] up or treat it in some gas to elevated temperatures.”
|
|
When the gold clusters are heated, the ligands start to come off and gold’s catalytic activity increases. The optimal temperature for producing gold nanocluster catalysts for carbon monoxide oxidation is 498 Kelvin (225 degrees Celsius or 437 degrees Fahrenheit), Wu said. If heating increases further, catalytic activity decreases because the gold particles become fluid and aggregate on the support.
|
|
Next the scientists are interested in varying the gold-cluster size and stabilizing the new clusters to make novel uniform catalysts. “We want to understand how other kinds of reactions can be catalyzed by these. So far we’ve only looked at carbon monoxide oxidation, which is kind of a test reaction,” Overbury said. “Our primary interest is using the gold-nanocluster complex as a toolbox for learning about how other complex reactions occur.”
|
|
Added Overbury, “We’re only just starting to mine all the catalytic possibilities for gold.”
|

