| Posted: Jul 22, 2016 |
Individual gold atoms can help generate clean fuel
(Nanowerk News) A combined experimental and theoretical study comprising researchers from the Chemistry Department and LCN, along with groups in Argentina, China, Spain and Germany, has shed new light on the behaviour of individual gold atoms supported on defective thin cerium dioxide films – an important system for catalysis and the generation of clean hydrogen for fuel.
|
|
Catalysis plays a vital role in our world; an estimated 80% of all chemical and materials are made via processes which involve catalysts, which are commonly a mixture of metals and oxides. The standard motif for these heterogeneous catalysts (where the catalysts are solid and the reactants are in the gas phase) is of a high surface area oxide support that is decorated with metal nanoparticles a few nanometres in diameter.
|
|
Cerium dioxide (ceria, CeO2) is a widely used support material for many important industrial processes; metal nanoparticles supported on ceria have displayed high activities for applications including car catalytic converters, alcohol synthesis, and for hydrogen production. There are two key attributes of ceria which make it an excellent active support material: its oxygen storage and release ability, and its ability to stabilise small metal particles under reaction conditions.
|
|
A recent system that has been the focus of much interest has been that of gold nanoparticles and single atoms with ceria, which has demonstrated high activity towards the water-gas-shift reaction, (CO + H2O ---> CO2 + H2) a key stage in the generation of clean hydrogen for use in fuel cells.
|
|
The nature of the active sites of these catalysts and the role that defects play are still relatively poorly understood; in order to study them in a systematic fashion, the researchers prepared model systems which can be characterised on the atomic scale with a scanning tunnelling microscope.
|
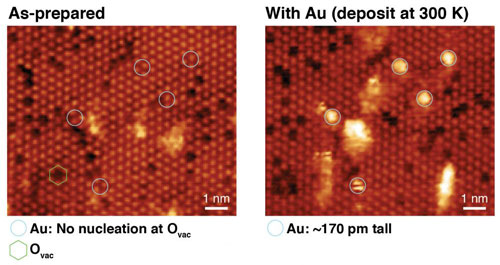 |
| STM images of CeO2-x(111) ultrathin films before and after the deposition of Au single atoms at 300 K. The bright lattice is from the oxygen atoms at the surface – vacancies appear as dark spots.
|
|
These model systems comprised well-ordered, epitaxial ceria films less than 2 nm thick, prepared on a metal single crystal, upon which single atoms and small clusters of gold were evaporated onto under ultra-high-vacuum (essential to prevent contamination of the surfaces). Oxygen vacancy defects – missing oxygen atoms in the top layer of the ceria – are relatively common at the surface and appear as dark spots in the STM images. By mapping the surface before and after the deposition of gold, it is possible to analyse the binding of the metal atoms, in particular there does not appear to be any preference for binding in the vacancy sites at 300 K.
|
|
Publishing their results in Physical Review Letters ("Diffusion Barriers Block Defect Occupation on Reduced CeO2(111)"), the researchers combined these experimental results with theoretical studies of the binding energies and diffusion rates across the surface. They showed that kinetic effects governed the behaviour of the gold atoms, prohibiting the expected occupation of the thermodynamically more stable oxygen vacancy sites.
|
|
They also identified electron transfer between the gold atoms and the ceria, leading to a better understanding of the diffusion phenomena that occur at this scale, and demonstrated that the effect of individual surface defects may be more minor than is normally imagined.
|
|
Related articles:
|
|
"Spillover Reoxidation of Ceria Nanoparticles" (2016) and "Spectromicroscopy of a Model Water–Gas Shift Catalyst: Gold Nanoparticles Supported on Ceria" (2014) both in The Journal of Physical Chemistry
|

