| Posted: Jul 24, 2017 |
Engineers invent the first bio-compatible, ion current battery
(Nanowerk News) Engineers at the University of Maryland have invented an entirely new kind of battery. It is bio-compatible, because it produces the same kind of electrical energy that the body uses.
|
|
In ordinary batteries the electrical energy, or current, delivered is in form of moving electrons. This flow of electrons out of the battery is generated by moving positive ions from one end to the other of the battery. This new device does the opposite. It moves electrons around in the device to deliver energy as a flow of ions out. This is how electricity is generated in the human body and all living things: through ion currents. It is the first time that an ionic current-generating battery has been invented.
|
|
“My intention is for ionic systems to interface with human systems,” said Liangbing Hu, the head of the group that developed that battery. Hu is a professor of materials science at the University of Maryland, College Park. He is also a member of the University of Maryland Energy Research Center and a principal investigator of the Nanostructures for Electrical Energy Storage Energy Frontier Research Center, sponsored by the Department of Energy, which funded the study ("Inverted battery design as ion generator for interfacing with biosystems").
|
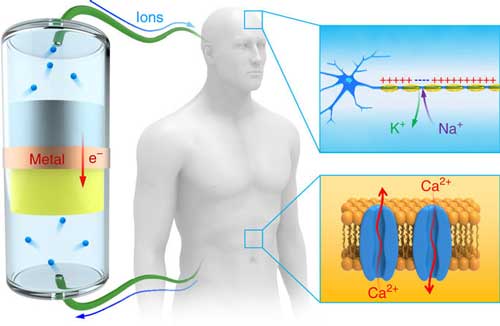 |
| Schematic of an electron battery for potential biological applications. In the human body, ions govern vital biological functions, such as signal transmittance in neurons, the movement of muscles and the activities of other cells. An electron battery composed of metal anodes and corresponding high voltage cathodes can continuously generate ions and power the motion of ions in various biosystems. Internally, the electron battery is connected by an electrical conductor that allows the transport of electrons, and its external electrodes are connected with the human body through ionic cables. (© NPG)
|
|
“So I came up with the reverse design of a battery. In a typical battery, electrons flow through wires to interface electronics, and ions flow through the battery separator. In our reverse design, a traditional battery is electronically shorted (that means electrons are flowing through the metal wires). Then ions have to flow through the outside ionic cables. In this case, the ions in the ionic cable – here, grass fibers -- can interface with living systems.”
|
|
“Potential applications might include the development of the next generation of devices to micro-manipulate neuronal activities and interactions that can prevent and/or treat such medical problems as Alzheimer’s disease and depression,” said Jianhua Zhang, PhD, a staff scientist at the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), part of the National Institutes of Health in Bethesda, Md.
|
|
Zhang performed biological experiments to test that the new battery transmitted current to living cells. “The battery could be used to develop medical devices for the disabled, or for more efficient drug and gene delivery tools in both research and clinical settings, as a way to more precisely treat cancers and other medical diseases,” he said. “Looking far ahead on the scientific horizon, one hopes also that this invention may help to establish the possibility of direct machine and human communication.”
|
|
The battery has another unusual feature – it uses grass to store its energy. To make the battery, the team soaked blades of Kentucky bluegrass in lithium salt solution. The channels that once moved nutrients up and down the grass blade were ideal conduits to hold the solution.
|
|
The battery looks like two glass tubes with a blade of grass inside each, connected by a thin metal wire at the top. The wire is where the electrons flow through to move from one end of the battery to the other as the stored energy slowly discharges. At the other end of each glass tube is a metal tip through which the ionic current flows.
|
|
They proved that the ionic current is flowing by touching the ends of the battery to either end of a lithium-soaked cotton string, with a dot of blue-dyed copper ions in the middle. Caught up in the ionic current, the copper moved along the string toward the negatively charged pole, just as the researchers predicted.
|
|
The team describes the example of calcium channels in the muscles and neural gaps in the brain — calcium ions move around in both. In fact, the body is a complex network of ion-current systems, sometimes using different ions to generate electrical fields in each organ system.
|
|
Because living cells work on an ionic current, scientists have previously tried to figure out how to patch an electronic current to an ionic current. The problem is that electronic current needs to reach a certain voltage to jump the gap between electronic systems and ionic systems. But living systems’ ionic currents flow at a very low voltage. So the electronic-induced current would be too high to, say, run a brain or a muscle. Ionic current, however, would flow as easily as a plug in a socket, and could be run at any voltage.
|
|
This is not the first time UMD scientists have tested natural materials in new uses. Hu and his team have been studying cellulose and plant materials for batteries, creating a battery and a supercapacitor out of wood and a battery from a leaf.
|
|
“To determine a good ionic cable, the conductivity needs to be high, while the mechanical property has to be strong,” said Chengwei Wang, first author of the paper and a graduate student in the Materials Science and Engineering department at the University of Maryland in College Park. “We also tried cotton string soaked with salt solution, which also has good mechanical strength and ionic conductivity. However, the salt solution easily evaporates because the solution is on the outside of the string. The microchannels in the grass can hold the salt solution, making them a stable ionic conductor.”
|
|
To test their battery in living cells, the researchers worked with Dr. Zhang of NIDDK. Zhang poked the ionic-current-emitting ends of the battery into a cell culture that generates fluorescent light when stimulated. After 30 minutes, all the cells in the culture had lit up, which means that the ionic current had successfully penetrated them.
|
|
“The invention of the ion current battery is a big leap forward, and it could revolutionize many aspects of medical science,” said Zhang.
|
|
The work is very creative and its main value is in delivering ionic flow to bio systems without posing other dangers to them, “ said Dr. Ping Liu, an associate professor in in nanoengineering at the University of California, San Diego, who was not involved with the study. “Eventually, the impact of the work really resides in whether smaller and more biocompatible junction materials can be found that then interface with cells and organisms more directly and efficiently.”
|
|
The team plans to diversify the types of ionic current electron batteries they can produce. “We are developing multiple ionic conductors with cellulose, hydrogels and polymers,” said Wang.
|

